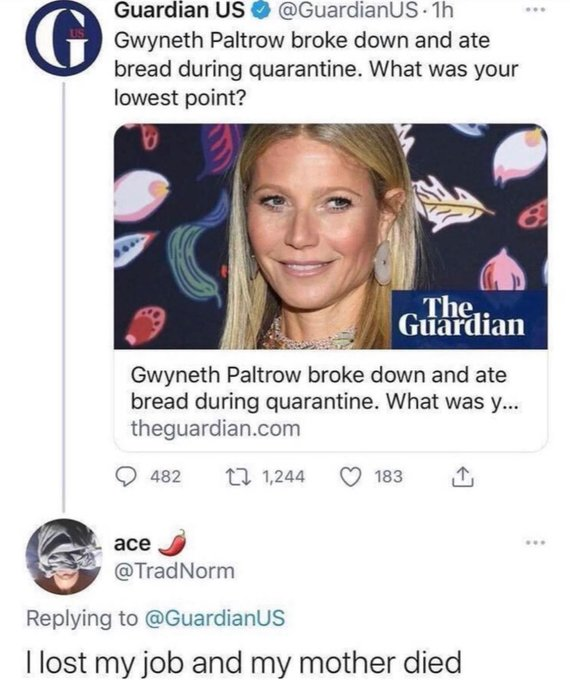
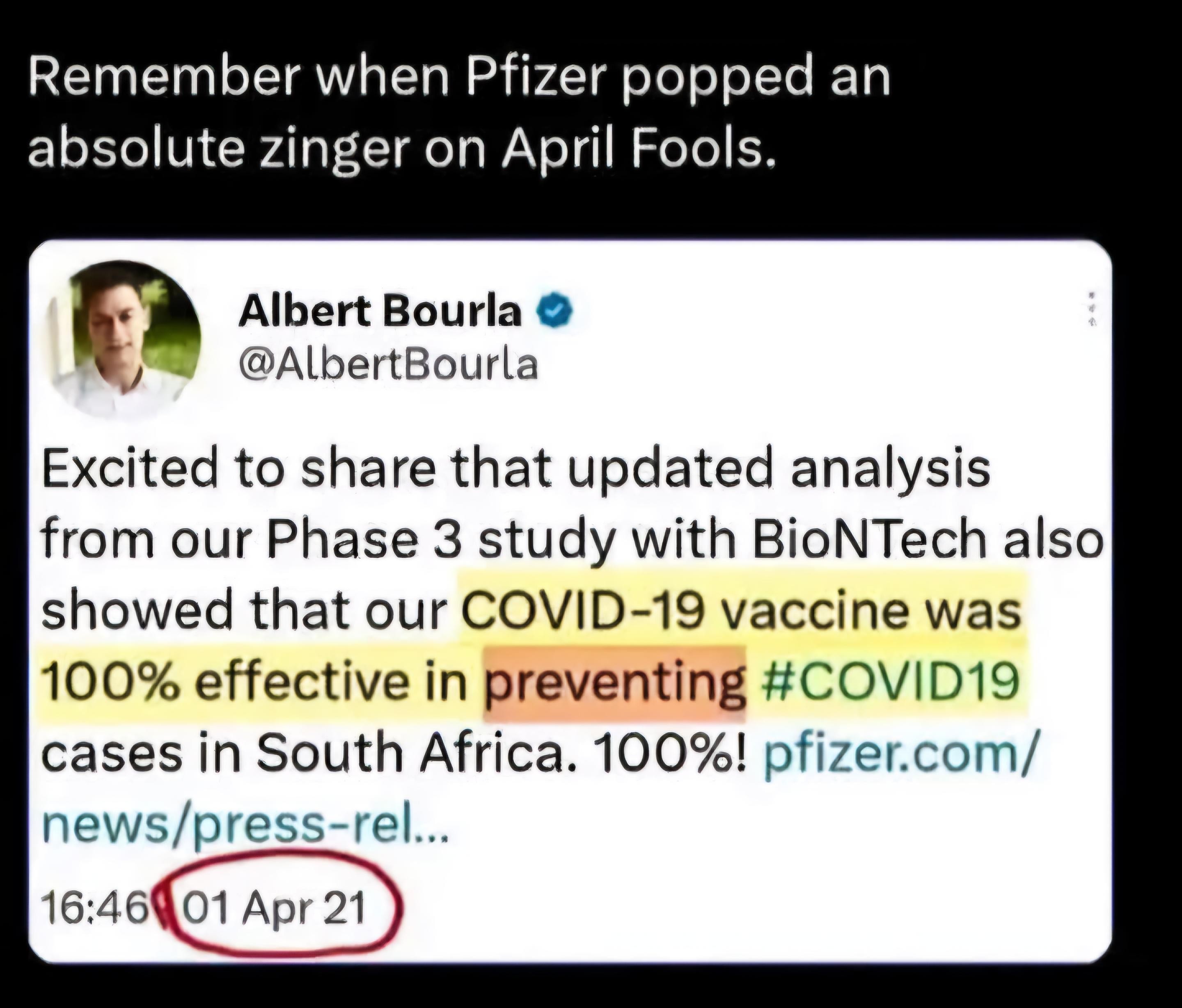
Yes. Anything that is scheduled and paid for on a national basis using tax $$$ is an incredibly powerful magnet for scammers. The most organized ones work in the pharmaceutical industry. At least the MIC crew produce stuff, although often it is not fit for purpose.seems to be a common thing...

Internal Emails Reveal Merck's Negligence in Gardasil Safety Testing ⋆ Brownstone Institute
A lawsuit against Merck is underway, marking company’s first jury trial over claims it misrepresented safety of Gardasil HPV vaccine.brownstone.org
Join the Hide community
Get access to live stream, lessons, the post exchange, and chat with other snipers.
Register
Download Gravity Ballistics
Get help to accurately calculate and scope your sniper rifle using real shooting data.

Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Died Suddenly Documentary World Is Live On Rumble
- Thread starter theLBC
- Start date
Another week, another dead congresscritter...
She was out in 2020.... But sheesh... these cancer cases are one a week! Meh, maybe that is normal background deaths for former congresscritters. But sure is making the news a lot!
Sirhr
She was out in 2020.... But sheesh... these cancer cases are one a week! Meh, maybe that is normal background deaths for former congresscritters. But sure is making the news a lot!
Sirhr

The Hospital Will Not Get Paid Their "Bonuses" if They Deviate From The "Treatment Plan"
Thomas Hospital, Alabama
This is massive! Not SCOTUS yet... but it's a big domino!
Sirhr
We have another to add to the death roll- 38 years old - none smoker - moderate drinker - has every vax and booster a dumb ass could get - had the WhuFlu falling out cause they went full Covid retard and we didn’t buy any of it - anyhow passed early this AM , swollen heart , several blood clots.
I am sorry this happened to you- but no refunds .
I am sorry this happened to you- but no refunds .
White House staff where are exempt from taking it. My guess is these are OD vs. suicide versus suicide by OD.
even if they were exempt, they probably still needed clotshots to eat out at restaurants and other things (depending on where they lived).White House staff where are exempt from taking it. My guess is these are OD vs. suicide versus suicide by OD.
I just heard of a 15 yr old who passed last week. Could be OD I guess. Not newsworthy though.
Or they were really scaredeven if they were exempt, they probably still needed clotshots to eat out at restaurants and other things (depending on where they lived).
They made up a phony birth certificate for the Kenyan. Handing out a few clot shot cards was like handing out candy from a PEZ dispensereven if they were exempt, they probably still needed clotshots to eat out at restaurants and other things (depending on where they lived).

Samsung Co-CEO Dies of Heart Attack at 63
Samsung’s co-CEO and vice chairman Han Jong-hee has died of a heart attack at 63. He was receiving treatment in the hospital for cardiac arrest on Tuesday when he died, a company spokesperson said. The TV industry expert joined Samsung Electronics Co.’s TV development team in 1988 after...
they don't mention that they were paid to push the lies.


The fuck you’re sorry you fucking blood soaked monsters. Fuck you, fuck your families, fuck anyone who sympathizes with you. Goddammit, I need to stop coming into this thread
don't know who these people are, but i am not doubting them.

Covid Vaccine: Truth and Justice from Trump? ⋆ Brownstone Institute
The Covid vaccine was a fix from the start, Americans deserve a full accounting of the risks behind the injection that Biden sought to compel.
 brownstone.org
brownstone.org

Nurses at Massachusetts hospital concerned about growing number of cancer cases among staff
Nurses at Newton-Wellesley Hospital say they're concerned about growing numbers of cancer cases among longtime nurses.

he belongs in prison.

 www.realclearpolicy.com
www.realclearpolicy.com

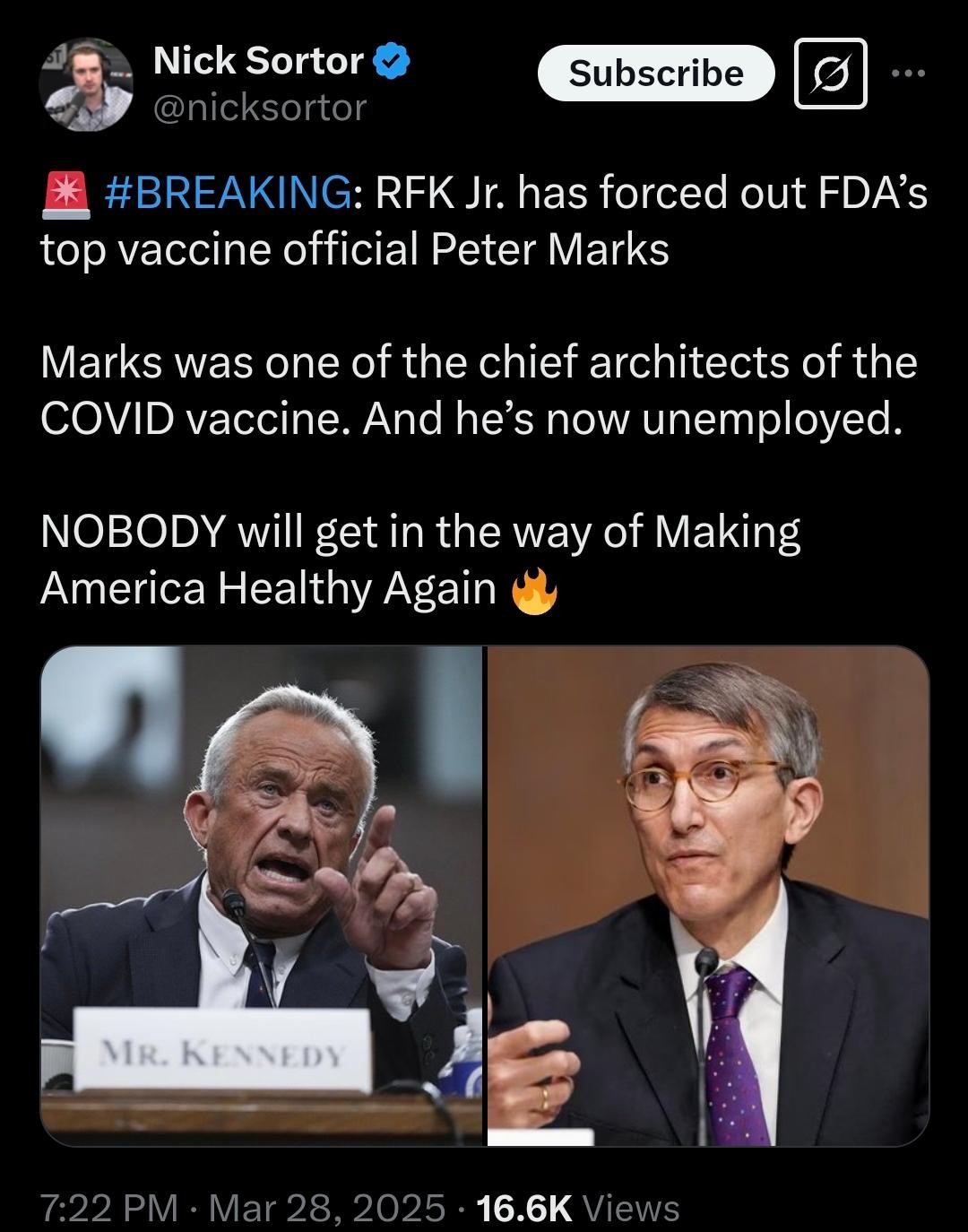
Follow the Science: Why Peter Marks Was Asked to Leave the FDA
On Friday, Dr. Peter Marks announced his resignation from the U.S. Food and Drug Administration (FDA) as Director of CEBR (Center for Biologics Evaluation and Research) citing differences with Health
On at least three additional, documented occasions during the Biden administration as Director of CBER, Marks disregarded the opinions and expert advice of long-time career scientists to advance his own dangerous agenda. In addition to ignoring and overruling FDA’s top vaccine scientists during the pandemic, Marks also overruled FDA career scientists and supported the approval of the Alzheimer’s drug ADUHELM; a decision later overturned. In 2023, he overruled his own staff scientists amid their concerns and those raised by an FDA Advisory Board to grant approval of ELEVIDYS, a gene treatment for Duchenne muscular dystrophy (DMD). Furthermore, in 2024 Marks expanded the approval of ELEVIDYS despite FDA staff objections and without FDA Advisory Committee input.
Last edited:
good thing she was fired, but that isn't enough. she needs to go to prison along with her scumbag liar husband.
Watch the short clip at the bottom folks.
Some individuals could run the risk of potentially producing spike proteins...permanently...!? WTF? I mean holy shit. That's the first time I've heard that. I figured it was a half life sort of a deal and would eventually go away.
Will anyone ever be brought to justice over this? Serious question...
NPCs and “good” Christians cannot seem to open their eyes and see the actual evil in our everyday lives. Like toddlers closing their eyes so adults can’t see them. The covid event is the seminal experience for white westerner society. Many remain willfully ignorant.Watch the short clip at the bottom folks.
Some individuals could run the risk of potentially producing spike proteins...permanently...!? WTF? I mean holy shit. That's the first time I've heard that. I figured it was a half life sort of a deal and would eventually go away.
Will anyone ever be brought to justice over this? Serious question...

mRNA “Vaccines” Linked to Genetic Changes That Can Cause Cancer, Autoimmune Disorders
A new peer-reviewed study links the mRNA COVID-19 vaccines to long-term changes in genetic structures that can provoke an inflammatory

'Consensus' – 97% of the French Academy of Medicine Agrees Covid Came from Wuhan Lab
Meanwhile, in a a justified retribution for ignoring real science and betraying the trust of the American people, thousands of public health bureaucrats have now been fired...including Dr. Anthony Fauci's wife (who was asked to transfer to Alaska).
Sad for the children, all because of the mental illness of their parents.
Early death will find them all and reduce the population of libtards and un-critical thinkers at the voting booth.
The real plan is to exterminate native born westerners and import the 3rd world as replacements.
i don't know if this shit is real, but if it was, you'd think it would be a bigger story.
imprison or execute 80% of the liberal elites in many fields for mass murder? doubt it.Watch the short clip at the bottom folks.
Some individuals could run the risk of potentially producing spike proteins...permanently...!? WTF? I mean holy shit. That's the first time I've heard that. I figured it was a half life sort of a deal and would eventually go away.
Will anyone ever be brought to justice over this? Serious question...
Well, what a fucking surprise. An experimental gene therapy that causes horrifying genetic changes…
mRNA “Vaccines” Linked to Genetic Changes That Can Cause Cancer, Autoimmune Disorders
A new peer-reviewed study links the mRNA COVID-19 vaccines to long-term changes in genetic structures that can provoke an inflammatorydiscernreport.com
You forgot all of team red that couldn’t get in line fast enough. Fuck them, tooimprison or execute 80% of the liberal elites in many fields for mass murder? doubt it.
Exactly like the animal testing done pre-covid.Well, what a fucking surprise. An experimental gene therapy that causes horrifying genetic changes…

More evidence of long-lasting & harmful COVID jab/spike protein
Y’all gonna need an exorcist.
Always remember that they basically lied to us about the jab and its products only being in the body for a few days, staying at the injection site, and doing no harm.
Okay then.
Similar threads
- Replies
- 19
- Views
- 1K