In Error, Tricare Tells 600K Beneficiaries They've Had COVID-19
Humana Military later corrected the correspondence soliciting convalescent plasma from coronavirus patients.
22 Jul 2020
Military.com | By Patricia Kime
More than 600,000 Tricare users in the military health system's East Region received emails Friday asking them to consider donating blood for research as "survivors of COVID-19."
But given that just 31,000 persons affiliated with the U.S. military have been diagnosed with the coronavirus, the email came as a surprise to beneficiaries.
Just wondering [if] anybody [got] an email from Tricare saying since you are a COVID survivor, please donate your plasma.?? I have NOT been tested," wrote a beneficiary on Facebook. "Just remember all those people inputting data are human and make mistakes."
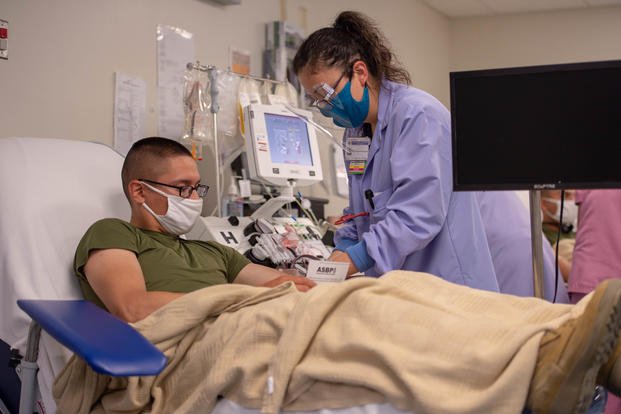
Humana Military, the company that manages Tricare for part or all of 31 states and the District of Columbia, issued a call to blood donors located near military installations that are collecting plasma from recovered coronavirus patients, also known as convalescent plasma, as a potential treatment for the illness.
But the message went to every beneficiary located near a collection point.
"As a survivor of COVID-19, it's safe to donate whole blood or blood plasma, and your donation could help other COVID-19 patients. Your plasma likely has antibodies (or proteins) present that might help fight the coronavirus infection. Currently, there is no cure for COVID-19. However, there is information that suggests plasma from COVID-19 survivors, like you, might help some patients recover more quickly from COVID-19," the email stated.
Six hours later, Humana issued a mea culpa.
"In an attempt to educate beneficiaries who live close to convalescent plasma donation centers about collection opportunities, you received an email incorrectly suggesting you were a COVID-19 survivor. You have not been identified as a COVID-19 survivor and we apologize for the error and any confusion it may have caused," the company wrote.
Marvin Hill, Humana's corporate communications lead, said the company apologizes "for the confusion caused by the original message," which was sent to recipients based on their proximity to a plasma collection facility and not "on any medical information or diagnosis."
"As a part of an effort to educate military beneficiaries about convalescent plasma donation opportunities, Humana was asked to assist our partner, the Defense Health Agency. Language used in email messages to approximately 600k beneficiaries gave the impression that we were attempting to reach only people who had tested positive for COVID-19. We quickly followed the initial email with a clear and accurate second message acknowledging this. We apologize," Hill said in a statement sent to Military.com.
The Defense Department announced in late May that it planned to collect 8,000 units of plasma by Sept. 30 from patients who recovered from COVID-19, part of a nationwide effort to study the effectiveness of convalescent plasma as a treatment for the illness.
But Friday, DoD officials said they have increased the goal to 10,000. The department has already collected 4,601 units.
"Our goal is to encourage all personnel who have fully recovered from COVID-19 to donate their convalescent plasma as a way to help their friends, family, or colleagues who may be suffering from the disease now or who may contract the disease in the future. The need is now," said Army Col. Audra Taylor, chief of the Armed Services Blood Program.
The U.S. Food and Drug Administration approved convalescent plasma as an investigational therapy in March for those hospitalized with the illness, and more than 35,000 patients in the U.S. have received it.
While there is no data that proves "definitively" that convalescent plasma works, there have been "encouraging reports and a lot of mechanistic reasoning that in fact convalescent plasma may be helpful," said Dr. Janet Woodcock, director of the Center for Drug Evaluation and Research at the FDA.
"These studies are being done as we speak ... we need donors. Blood drives are ongoing, and the U.S. government will be trying to accelerate these drives for convalescent plasma," Woodcock said in a call with reporters last week.
Antibodies found in convalescent plasma also serve as a source for another potential treatment for COVID-19: monoclonal antibodies. These are clones of isolated neutralizing antibodies -- either taken from humans or artificially created -- that are currently in development and safety testing as an "antibody cocktail" to treat the coronavirus for patients both in and out of hospitals, according to Woodcock.
In addition to collecting convalescent plasma at 15 military blood donation centers, DoD plans to deploy a mobile blood collection unit to areas with a high concentration of COVID-19 cases, Taylor said.
To be eligible, donors must be at least 17 years old, weigh at least 110 lbs., be in good health and be symptom-free for 14 days. Additionally, potential donors must have a prior diagnosis of COVID-19.
As of Monday, 31,418 patients affiliated with the Defense Department have tested positive for COVID-19, including 21,909 service members. Nationwide, nearly 3.9 million Americans have contracted the virus and 141,426 have died.
Worldwide, since the coronavirus was first detected in China in December, 14.7 million people have had confirmed cases and 611,599 have died, according to Johns Hopkins University.
-- Military.com Staff Writer Oriana Pawlyk contributed to this report.
-- Patricia Kime can be reached at [email protected]. Follow her on Twitter @patriciakime.

