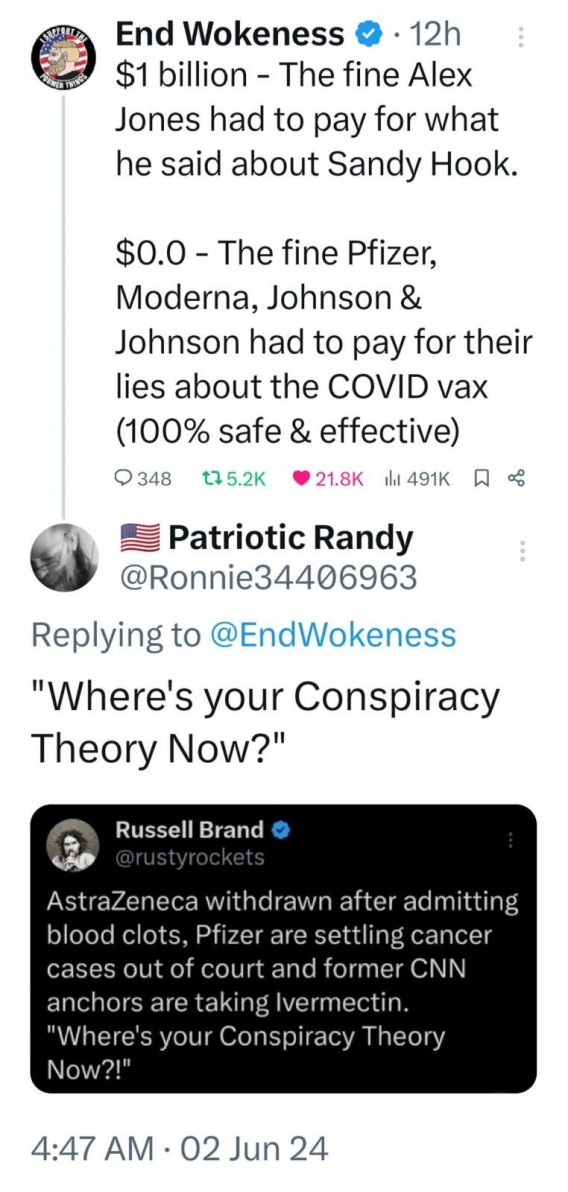
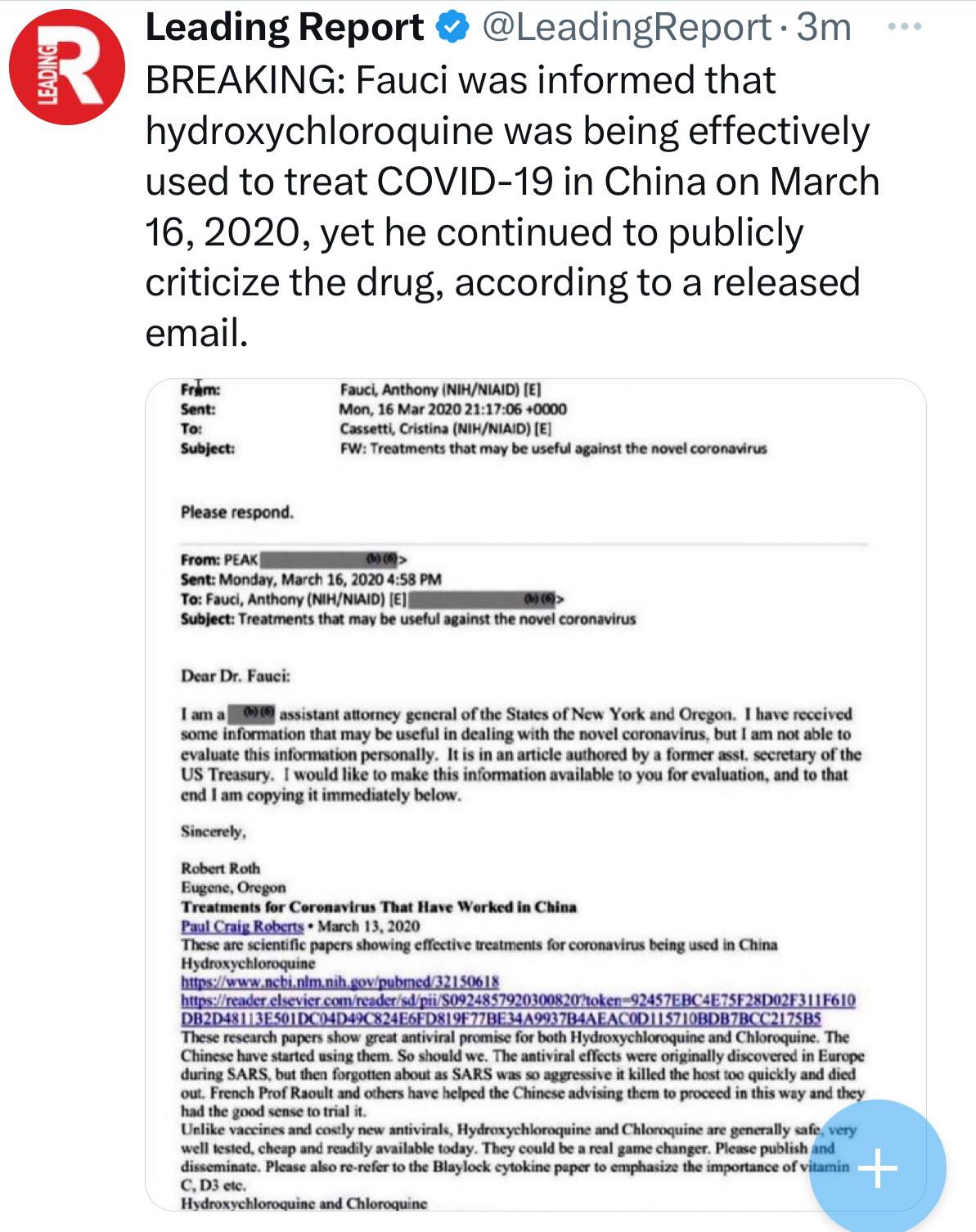
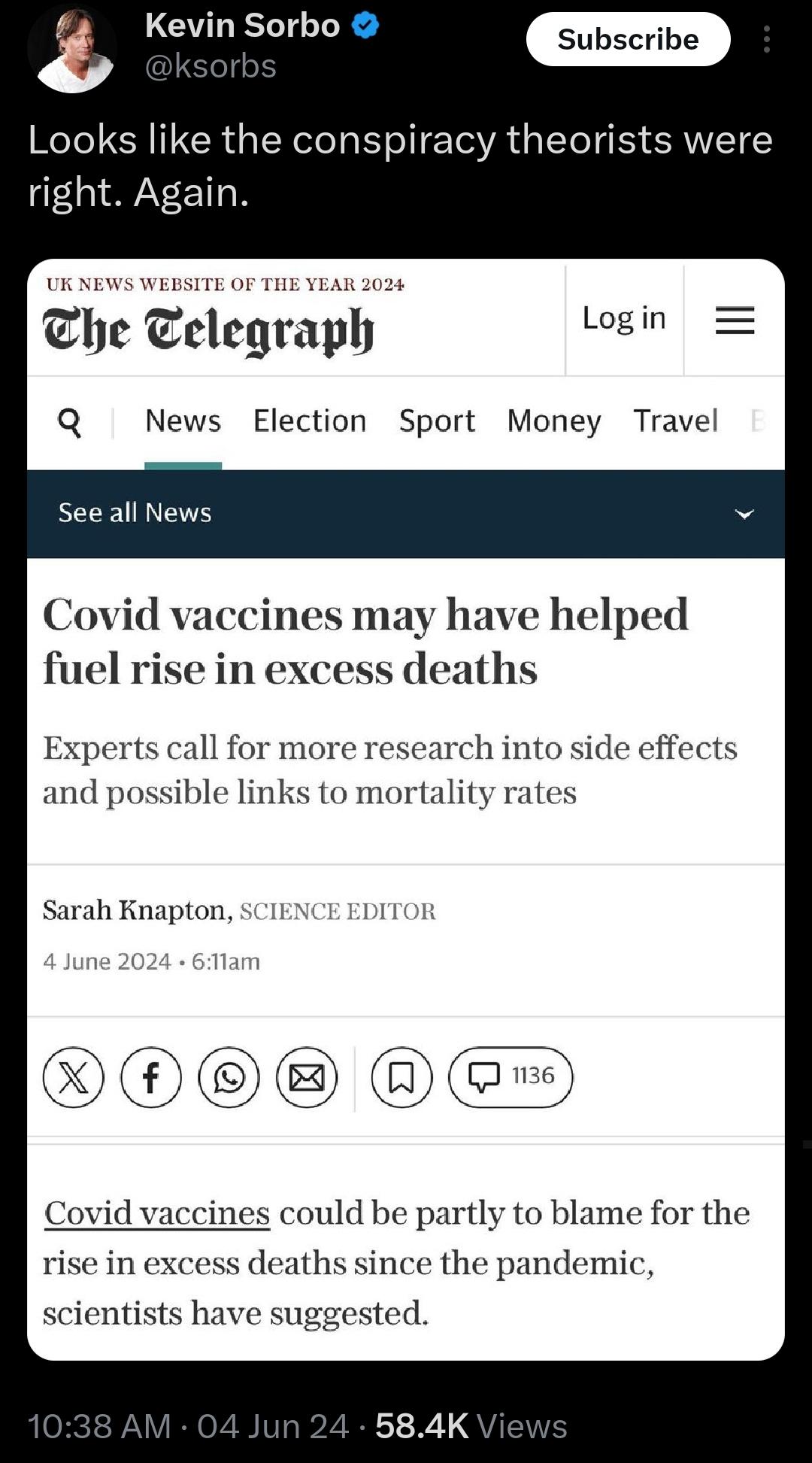
Jackson, a 20-year veteran in clinical trial administration employed by a third-party vendor (Ventavia Research Group), worked on Pfizer’s COVID-19 vaccine trials in 2020. Alarmed by what she witnessed, Jackson raised concerns to her superiors, Pfizer, and the Food and Drug Administration (FDA) in September 2020.
She claimed the trial was being run, documented, and reported in a manner that violated Federal law and was potentially dangerous.
Hours after contacting the FDA on September 25, 2020, Jackson was fired. Her sealed whistleblower complaint seemed to stall, with the FDA not investigating her claims. Faced with inaction, Jackson filed a lawsuit.
As the case progressed towards discovery, the DOJ intervened, asking the judge to dismiss the case. Jackson argues that the government failed to articulate a legitimate reason for dismissal and did not demonstrate why the burdens of continued litigation outweigh its benefits.
Disturbingly, a former FDA lawyer who worked at the agency when Jackson’s complaint was filed has moved to the DOJ and is now representing the government in its attempt to shut down the suit, raising concerns about regulatory capture and the use of government to shield companies from accountability.
Brooke Jackson did not kill herself.
Just getting ahead of the curve.
Sirhr