You guys are going to really need to get this one - this Marburg here has a 90% mortality. Just spread from New Guinea to Tanzania. But NO WORRIES folks, BioNTech, the same awesome innovators that brought us the Covid vaccine with 95% efficacy, has already a superb mRNA Marburg vaccine ready to go as of end of January. Plus, the NIH has done its first human trials in January with Marburg vaccines at Walter Reed.
And - low and behold - an outbreak of Marburg in New Guinea in February just happened!!! What are the odds!!
Published end of January:
https://www.linkedin.com › pulse › vaccine-production-marburg-has-right-stuff-christian-korner
+ Follow
How quickly “normal life” can be resumed depends in part on how quickly the population can be vaccinated. One of the vaccinations that provides particularly good protection against Covid-19 is the BNT162b2 vaccine from Mainz-based biotech company BioNTech SE. To scale-up the manufacturing capacity further, the company began production in Marburg which is one of the largest mRNA-based vaccine manufacturing sites worldwide. To achieve this, BioNTech drew on the expertise available at Siemens to enable it to provide more of the sought-after vaccine as quickly as possible.
BioNTech manufactures BNT162b2 in collaboration with US pharmaceutical specialist Pfizer. The company has started manufacturing in Marburg, in the German state of Hesse. In the fall of 2020, it took over a production facility for biotech products from Novartis for this purpose. That gives it a good basis for successful vaccine production: The plant at Marburg comes with an ultramodern production facility for recombinant proteins. The relevant expertise is also available, since BioNTech also acquired a highly qualified employee base along with the production facility, all of whom are experienced in developing new technologies. The facility in Marburg had been producing influenza vaccines based on flu cell culture, then changed over to recombinant proteins for cancer treatments and now manufactures mRNA vaccine. Siemens is providing support with the switchover to vaccine production. There are two reasons for this: Siemens already knows the Marburg facility very well and has worked with it previously on activities including automation of vaccine production development. The two companies can also look back on a number of projects they have already completed together in the previous years.

Full speed ahead thanks to total commitment
Everyone involved in the project knew how important it was: The sooner production could start; the sooner more vaccine doses will be available. The hopes of society therefore depend on this project: German Chancellor Angela Merkel even commented at the Federal Press Conference on February 1, 2021, that “Marburg will make the difference.” A project of this magnitude normally takes about a year, but in this case the participants completed the conversion in just five months. Main components of the new manufacturing execution system (MES) were completed in only 2.5 months. Andreas Haag, in charge of the project for Siemens, observes: “One factor that made it possible was the high level of cooperation between the teams and outstanding commitment by everyone involved: They all worked overtime and took no leave in order to advance the project as fast as possible.” And under pandemic conditions, too: Things that would normally be done by working together on-site were instead performed largely by a decentralized team based in separate home offices. The project was a complete success, since the new Electronic Batch Records (eBPR) as part of the manufacturing execution system (MES) was ready to use in just 2.5 months.
What was converted?
During the project to switch the Marburg plant over to production of mRNA-based vaccines, Siemens focused on future viability. All the improvements are Industrie 4.0-compatible. One of the challenges with the conversion was the fact that it involved switching from rigid to mobile production with many single-use components. For example, it meant a whole raft of components had to be registered. That was one reason why the project partners decided to switch to paperless production. At the same time, working with mRNA meant a higher clean room class than was previously required in the facility. Paper is now an avoidable “contamination factor” that doesn’t arise with digital production. That was the basis for opting for the Opcenter Execution Pharma solution from Siemens as the new MES. This solution enables complete paperless manufacturing and fully electronic batch recording. Seamlessly integrating automation solutions makes it possible to develop, optimize, and manage production processes automatically. Because mRNA processes include a lot of manual stages – weighing, for example – operators require guidance through these. This is provided by the workflow management component of the software. Opcenter Execution Pharma orchestrates the various sections of the system to ensure efficient production. The software enables real-time production and the provision and analysis of process and quality information in order to optimize production activities from initial order to finished product.
To automate the facility, all systems were converted to the latest version of Simatic PCS 7. The powerful, flexible, and scalable distributed control system steers and controls all the processes in the plant and takes digitalization to field level. Although most parts of the new MES are now in place, additional automation components are still in progress.
In focus: paperless production
Paperless production offers advantages over traditional procedures in the pharmaceutical industry in particular: Process data, conditions, and results are recorded in detail to ensure processes are more resistant to error, in other words, they are made more robust and less susceptible to deviations. At the same time, the tasks of data input and documentation are now less complex. Electronic Master Batch Record Management enables users to create, execute, review, and release Master Batch Records (MBR), and Electronic Batch Records (eBR) are made faster. The individuals in charge can easily monitor, observe and, if necessary, record every stage of production and every base material. Testing is based on the principle of “review by exception” – in other words, deviations are dealt with when the system recognizes them based on exception rules. That makes the testing process less labor-intensive and much faster, since otherwise the individuals in charge would have to check several thousand pages on paper. As a result, digital production is a significant factor in making the process faster and improving quality.
Unity is strength
To ensure a smooth start to production, Siemens supported the implementation of the system at BioNTech with Hypercare and a 24/7 project-based standby arrangement. That means the employees in production could request help with operating the system from the manufacturer at any time of the day or night. The project is a complete success for both parties and the production was able to start before the end of February with the production of the drug substance: the mRNA. “We want to thank Siemens for their excellent collaboration on this project and the huge effort they put in, often exceeding 100 percent,” says Valeska Schilling, Head of Production Department at BioNTech Marburg.



 62
62
 Colorized scanning electron micrograph of Marburg virus particles (blue) both budding and attached to the surface of infected VERO E6 cells (orange).
Colorized scanning electron micrograph of Marburg virus particles (blue) both budding and attached to the surface of infected VERO E6 cells (orange).
This first-in-human, Phase 1 study tested an experimental MARV vaccine candidate, known as cAd3-Marburg, which was developed at NIAID’s Vaccine Research Center (VRC). This vaccine uses a modified chimpanzee adenovirus called cAd3, which can no longer replicate or infect cells, and displays a glycoprotein found on the surface of MARV to induce immune responses against the virus. The cAd3 vaccine platform demonstrated a good safety profile in prior clinical trials when used in investigational Ebola virus and Sudan virus vaccines developed by the VRC.
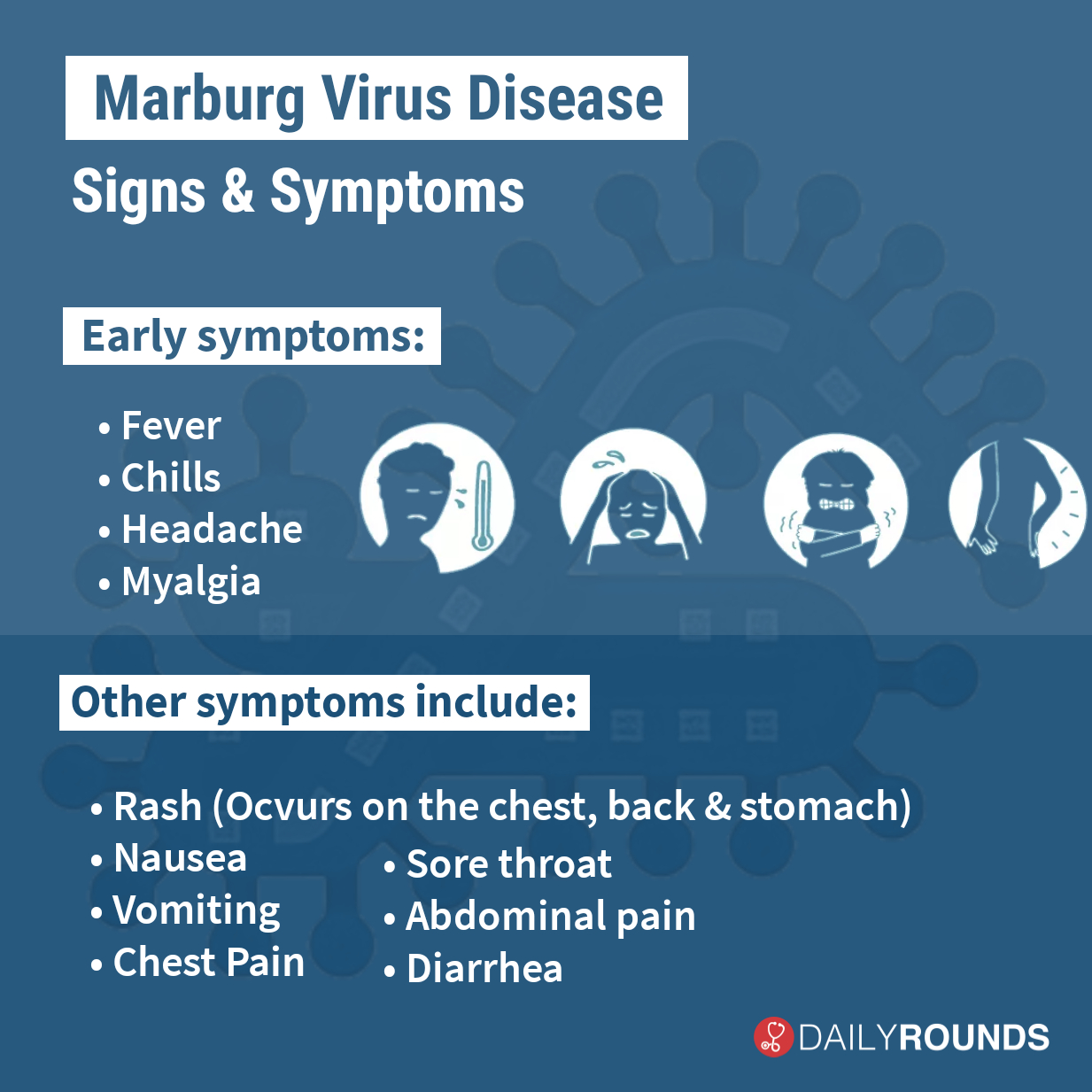
MARV, a filovirus in the same family as Ebola virus, causes a rapidly progressive febrile illness that leads to shock and death in a large proportion of infected individuals. Many scientists think that MARV disease outbreaks in humans begin by when the virus makes the jump from its primary animal host, which is likely to be certain chronically infected bats in sub-Saharan Africa. The symptoms of MARV disease are akin to those seen with Ebola virus disease and can include fever, headache, chills, rash, abdominal pain, vomiting, and diarrhea. As the disease progresses, patients may suffer from multiple organ dysfunction, delirium, and significant bleeding from the gastrointestinal tract or other sites that may result in death. No approved vaccines or specific therapies are available for MARV disease, aside from supportive care. While some experimental vaccines have previously been tested, none have proven to be both highly effective and to provide durable protection. In areas of Africa where a vaccine for Marburg is most needed, a single-dose vaccine that could protect recipients over a long period of time would be a crucial part of quelling outbreaks.
In this study, 40 healthy adult volunteers were enrolled at the Walter Reed Army Institute of Research Clinical Trials Center in Silver Spring, Maryland. They received a single dose of either a low dose of the vaccine (1x1010 particle units) or a higher dose (1x1011 particle units). For safety, the volunteers were enrolled in a dose-escalation plan. Three participants received the lower dose. Then, when they did not exhibit severe adverse reactions after the first seven days, the trial proceeded to enroll the remaining 17 volunteers. The same procedure was also used for the higher dose group. Volunteers were monitored for adverse reactions to the investigational vaccine and evaluated at regular intervals for 48 weeks to track their immune responses.
The trial’s safety results were encouraging: There were no serious adverse events, and the experimental vaccine was well-tolerated. One participant in the higher dose group developed a fever following vaccination, but it resolved by the following day. In addition, the investigational vaccine appeared to induce strong, long-lasting immunity to the MARV glycoprotein: 95% of participants in the trial exhibited a robust antibody response after vaccination, and 70% maintained that response for more than 48 weeks.
Plans are in place to conduct further trials of the cAd3-Marburg vaccine in Ghana, Kenya, Uganda, and the United States. If additional data supports the promising results seen in the Phase 1 trial, the cAd3-Marburg virus vaccine could someday be used in emergency responses to MARV outbreaks.
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
--------------

Duration 2:13
There's a race to contain an outbreak of Marburg disease — caused by an Ebola-related virus — in Equatorial Guinea, where at least nine people have died. There's no cure for the deadly disease and development of a vaccine was paused years ago.
The World Health Organization this week confirmed an outbreak of the Marburg virus in Equatorial Guinea — the first time the tiny country in Central Africa has seen cases of the deadly illness.
Marburg, which is related to Ebola, is already being blamed for at least nine deaths in the country, and another 16 suspected cases are being investigated.
Without treatment, Marburg can be fatal in up to 88 per cent of people.
A 2004-05 outbreak in Angola killed 90 per cent of the 252 confirmed cases.
Here is what you need to know about this rare but dangerous virus.
According to the World Health Organization (WHO), people can contract the virus through prolonged exposure in mines or caves where the bat colonies live.
The virus spreads between humans through direct contact with blood or other bodily fluids of an infected individual, or with surfaces contaminated with the virus, such as clothing or bed sheets.
Marburg is not airborne.

Marburg virus is believed to have originated in the African fruit bat, and it can be contracted by humans through prolonged exposure to mines or caves where the animal lives. (Bob Child/The Associated Press)
"It can impact every organ, and it essentially will cause a shock-like syndrome," said Dr. Isaac Bogoch, an infectious diseases specialist at Toronto General Hospital.
He said the virus can also cause gastrointestinal complications and a predilection to easy bleeding.
WHO says a rash can appear in the first seven days, and the central nervous system can be affected, resulting in confusion, aggression and irritability.
If death occurs, it generally happens eight to nine days after onset, following severe blood loss and shock.
An electron microscope photo of the Marburg virus. An outbreak of the deadly virus has been confirmed in the Central African country of Equatorial Guinea. (Thomas Geisbert/University of Texas Medical Branch)
"They need supportive care," Bogoch said, including intravenous fluids, as well as electrolyte balance and monitoring. "That can significantly lower the mortality rate," he said.
The outbreak was confirmed after samples were sent to a lab in Senegal. Suspected cases from Cameroon and Gabon were also investigated but found not to be Marburg, WHO said.
But that doesn't mean there aren't more Marburg cases.
"When new diseases appear in new locations, we are often just seeing a piece of the picture," said Dr. Kamran Khan, the founder and CEO of Toronto-based BlueDot, a company that tracks infectious diseases around the world.
He said there are probably more cases and more contacts than the official numbers would indicate, noting that Equatorial Guinea is one of the most resource limited countries in the world.
"Its capabilities in terms of its health-care system, its public health infrastructure for countering an outbreak, are pretty limited," he said.
WHO said it is sending medical experts to help local officials in Equatorial Guinea, along with protective equipment for hundreds of workers.
"Surveillance in the field has been intensified," said George Ameh, WHO's country representative in Equatorial Guinea.
"Contact tracing, as you know, is a cornerstone of the response. We have ... redeployed the COVID-19 teams that were there for contact tracing and quickly retrofitted them to really help us out."
WHO director general Tedros Adhanom Ghebreyesus said the agency is also supporting the governments of Cameroon and Gabon "to prepare, to rapidly detect, isolate and provide care for any suspected cases."
WATCH | WHO is deploying teams to Equatorial Guinea to deal with Marburg outbreak:

Duration 0:56
WHO director general Tedros Adhanom Ghebreyesus says the agency is sending teams and supplies to the Central African country of Equatorial Guinea to try to contain the current outbreak of the deadly Marburg virus.
"We saw that with, for example, the West African Ebola virus epidemic — which started off as a very small outbreak, turned into a multi-country, multi-year outbreak that took a long time to get under control."
Khan of BlueDot said Equatorial Guinea is going to need international assistance to be able to get ahead of this outbreak. "Today, it's a concern for the region and some of the neighbouring countries. But if we don't get ahead of this, this could become a broader concern for the global community."
"I think this is important for Canadians to understand that the likelihood of a case of Marburg showing up in Canada right now is exceedingly low," Kahn said. But he said it's important to be aware of the larger issue — which is that "there are more outbreaks appearing in the world today, they are becoming larger, they are becoming more dangerous and disruptive."
Bogoch said while the current Marburg outbreak is small, now is the time to jump on it so that it doesn't expand.
--------------------------------------------------------
Well, its already to Tanzania, but obviously the Canadian press weren't on top of how our astute virologists saw this coming and were doing the first vaccine clinical trials in January. And the head dude at Siemens was appropriately proud of this as can be seen in his January 25 article above.
Just amazing, almost like these guys had a crystal ball. Thank goodness the WHO is finally (or will be within a week or so) in charge of everything so we don't have to worry about local bureacracies or even the wisdom of supersmart Joe BIden interfering one bit!
And - low and behold - an outbreak of Marburg in New Guinea in February just happened!!! What are the odds!!
Published end of January:
https://www.linkedin.com › pulse › vaccine-production-marburg-has-right-stuff-christian-korner
Vaccine production: Marburg has the right stuff
Christian KornerChristian Korner
Head of Pharma Business US at Siemens
Published Jan 25, 2023+ Follow
How quickly “normal life” can be resumed depends in part on how quickly the population can be vaccinated. One of the vaccinations that provides particularly good protection against Covid-19 is the BNT162b2 vaccine from Mainz-based biotech company BioNTech SE. To scale-up the manufacturing capacity further, the company began production in Marburg which is one of the largest mRNA-based vaccine manufacturing sites worldwide. To achieve this, BioNTech drew on the expertise available at Siemens to enable it to provide more of the sought-after vaccine as quickly as possible.
BioNTech manufactures BNT162b2 in collaboration with US pharmaceutical specialist Pfizer. The company has started manufacturing in Marburg, in the German state of Hesse. In the fall of 2020, it took over a production facility for biotech products from Novartis for this purpose. That gives it a good basis for successful vaccine production: The plant at Marburg comes with an ultramodern production facility for recombinant proteins. The relevant expertise is also available, since BioNTech also acquired a highly qualified employee base along with the production facility, all of whom are experienced in developing new technologies. The facility in Marburg had been producing influenza vaccines based on flu cell culture, then changed over to recombinant proteins for cancer treatments and now manufactures mRNA vaccine. Siemens is providing support with the switchover to vaccine production. There are two reasons for this: Siemens already knows the Marburg facility very well and has worked with it previously on activities including automation of vaccine production development. The two companies can also look back on a number of projects they have already completed together in the previous years.

Full speed ahead thanks to total commitment
Everyone involved in the project knew how important it was: The sooner production could start; the sooner more vaccine doses will be available. The hopes of society therefore depend on this project: German Chancellor Angela Merkel even commented at the Federal Press Conference on February 1, 2021, that “Marburg will make the difference.” A project of this magnitude normally takes about a year, but in this case the participants completed the conversion in just five months. Main components of the new manufacturing execution system (MES) were completed in only 2.5 months. Andreas Haag, in charge of the project for Siemens, observes: “One factor that made it possible was the high level of cooperation between the teams and outstanding commitment by everyone involved: They all worked overtime and took no leave in order to advance the project as fast as possible.” And under pandemic conditions, too: Things that would normally be done by working together on-site were instead performed largely by a decentralized team based in separate home offices. The project was a complete success, since the new Electronic Batch Records (eBPR) as part of the manufacturing execution system (MES) was ready to use in just 2.5 months.
What was converted?
During the project to switch the Marburg plant over to production of mRNA-based vaccines, Siemens focused on future viability. All the improvements are Industrie 4.0-compatible. One of the challenges with the conversion was the fact that it involved switching from rigid to mobile production with many single-use components. For example, it meant a whole raft of components had to be registered. That was one reason why the project partners decided to switch to paperless production. At the same time, working with mRNA meant a higher clean room class than was previously required in the facility. Paper is now an avoidable “contamination factor” that doesn’t arise with digital production. That was the basis for opting for the Opcenter Execution Pharma solution from Siemens as the new MES. This solution enables complete paperless manufacturing and fully electronic batch recording. Seamlessly integrating automation solutions makes it possible to develop, optimize, and manage production processes automatically. Because mRNA processes include a lot of manual stages – weighing, for example – operators require guidance through these. This is provided by the workflow management component of the software. Opcenter Execution Pharma orchestrates the various sections of the system to ensure efficient production. The software enables real-time production and the provision and analysis of process and quality information in order to optimize production activities from initial order to finished product.
To automate the facility, all systems were converted to the latest version of Simatic PCS 7. The powerful, flexible, and scalable distributed control system steers and controls all the processes in the plant and takes digitalization to field level. Although most parts of the new MES are now in place, additional automation components are still in progress.
In focus: paperless production
Paperless production offers advantages over traditional procedures in the pharmaceutical industry in particular: Process data, conditions, and results are recorded in detail to ensure processes are more resistant to error, in other words, they are made more robust and less susceptible to deviations. At the same time, the tasks of data input and documentation are now less complex. Electronic Master Batch Record Management enables users to create, execute, review, and release Master Batch Records (MBR), and Electronic Batch Records (eBR) are made faster. The individuals in charge can easily monitor, observe and, if necessary, record every stage of production and every base material. Testing is based on the principle of “review by exception” – in other words, deviations are dealt with when the system recognizes them based on exception rules. That makes the testing process less labor-intensive and much faster, since otherwise the individuals in charge would have to check several thousand pages on paper. As a result, digital production is a significant factor in making the process faster and improving quality.
Unity is strength
To ensure a smooth start to production, Siemens supported the implementation of the system at BioNTech with Hypercare and a 24/7 project-based standby arrangement. That means the employees in production could request help with operating the system from the manufacturer at any time of the day or night. The project is a complete success for both parties and the production was able to start before the end of February with the production of the drug substance: the mRNA. “We want to thank Siemens for their excellent collaboration on this project and the huge effort they put in, often exceeding 100 percent,” says Valeska Schilling, Head of Production Department at BioNTech Marburg.



 62
62-------------------------------------------------------------------
Marburg vaccine shows promising results in first-in-human study

What
A newly published paper in The Lancet shows that an experimental vaccine against Marburg virus (MARV) was safe and induced an immune response in a small, first-in-human clinical trial. The vaccine, developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, could someday be an important tool to respond to Marburg virus outbreaks.This first-in-human, Phase 1 study tested an experimental MARV vaccine candidate, known as cAd3-Marburg, which was developed at NIAID’s Vaccine Research Center (VRC). This vaccine uses a modified chimpanzee adenovirus called cAd3, which can no longer replicate or infect cells, and displays a glycoprotein found on the surface of MARV to induce immune responses against the virus. The cAd3 vaccine platform demonstrated a good safety profile in prior clinical trials when used in investigational Ebola virus and Sudan virus vaccines developed by the VRC.
MARV, a filovirus in the same family as Ebola virus, causes a rapidly progressive febrile illness that leads to shock and death in a large proportion of infected individuals. Many scientists think that MARV disease outbreaks in humans begin by when the virus makes the jump from its primary animal host, which is likely to be certain chronically infected bats in sub-Saharan Africa. The symptoms of MARV disease are akin to those seen with Ebola virus disease and can include fever, headache, chills, rash, abdominal pain, vomiting, and diarrhea. As the disease progresses, patients may suffer from multiple organ dysfunction, delirium, and significant bleeding from the gastrointestinal tract or other sites that may result in death. No approved vaccines or specific therapies are available for MARV disease, aside from supportive care. While some experimental vaccines have previously been tested, none have proven to be both highly effective and to provide durable protection. In areas of Africa where a vaccine for Marburg is most needed, a single-dose vaccine that could protect recipients over a long period of time would be a crucial part of quelling outbreaks.
In this study, 40 healthy adult volunteers were enrolled at the Walter Reed Army Institute of Research Clinical Trials Center in Silver Spring, Maryland. They received a single dose of either a low dose of the vaccine (1x1010 particle units) or a higher dose (1x1011 particle units). For safety, the volunteers were enrolled in a dose-escalation plan. Three participants received the lower dose. Then, when they did not exhibit severe adverse reactions after the first seven days, the trial proceeded to enroll the remaining 17 volunteers. The same procedure was also used for the higher dose group. Volunteers were monitored for adverse reactions to the investigational vaccine and evaluated at regular intervals for 48 weeks to track their immune responses.
The trial’s safety results were encouraging: There were no serious adverse events, and the experimental vaccine was well-tolerated. One participant in the higher dose group developed a fever following vaccination, but it resolved by the following day. In addition, the investigational vaccine appeared to induce strong, long-lasting immunity to the MARV glycoprotein: 95% of participants in the trial exhibited a robust antibody response after vaccination, and 70% maintained that response for more than 48 weeks.
Plans are in place to conduct further trials of the cAd3-Marburg vaccine in Ghana, Kenya, Uganda, and the United States. If additional data supports the promising results seen in the Phase 1 trial, the cAd3-Marburg virus vaccine could someday be used in emergency responses to MARV outbreaks.
Article
M Hamer et al. Safety, tolerability, and immunogenicity of the Marburg chimpanzee adenovirus vector vaccine (cAd3-Marburg) in healthy adults: a phase 1, open-label, dose-escalation trial. The Lancet DOI: 10.1016/S0140-6736(22)02400-X (2023).Who
Lesia Dropulic, M.D., chief of the Clinical Trials Program at NIAID’s Vaccine Research Center, is available for comment.Contact
To schedule interviews, please contact Elizabeth Deatrick, (301) 402-1663, [email protected].NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
--------------
An outbreak of the deadly Marburg virus has been confirmed. Here's what you need to know
There is no vaccine or drug treatment for the virus, which has killed at least 9 people in Equatorial Guinea
Stephanie Hogan · CBC News · Posted: Feb 17, 2023 3:00 AM CST | Last Updated: February 18
Racing to contain Marburg virus outbreak in Equatorial Guinea
1 month agoDuration 2:13
There's a race to contain an outbreak of Marburg disease — caused by an Ebola-related virus — in Equatorial Guinea, where at least nine people have died. There's no cure for the deadly disease and development of a vaccine was paused years ago.
The World Health Organization this week confirmed an outbreak of the Marburg virus in Equatorial Guinea — the first time the tiny country in Central Africa has seen cases of the deadly illness.
Marburg, which is related to Ebola, is already being blamed for at least nine deaths in the country, and another 16 suspected cases are being investigated.
Without treatment, Marburg can be fatal in up to 88 per cent of people.
A 2004-05 outbreak in Angola killed 90 per cent of the 252 confirmed cases.
Here is what you need to know about this rare but dangerous virus.
What is Marburg virus?
Marburg virus is believed to have originated in African fruit bats. It was first identified in 1967 in Germany and the former Yugoslavia, according to the U.S. Centers for Disease Control and Prevention, among people who had been working with green monkeys that had been imported from Uganda.According to the World Health Organization (WHO), people can contract the virus through prolonged exposure in mines or caves where the bat colonies live.
The virus spreads between humans through direct contact with blood or other bodily fluids of an infected individual, or with surfaces contaminated with the virus, such as clothing or bed sheets.
Marburg is not airborne.

Marburg virus is believed to have originated in the African fruit bat, and it can be contracted by humans through prolonged exposure to mines or caves where the animal lives. (Bob Child/The Associated Press)
What are the symptoms?
Symptoms may begin "abruptly," according to WHO, and include high fever, severe headache and malaise. Muscle aches and pains are also common."It can impact every organ, and it essentially will cause a shock-like syndrome," said Dr. Isaac Bogoch, an infectious diseases specialist at Toronto General Hospital.
He said the virus can also cause gastrointestinal complications and a predilection to easy bleeding.
WHO says a rash can appear in the first seven days, and the central nervous system can be affected, resulting in confusion, aggression and irritability.
If death occurs, it generally happens eight to nine days after onset, following severe blood loss and shock.

An electron microscope photo of the Marburg virus. An outbreak of the deadly virus has been confirmed in the Central African country of Equatorial Guinea. (Thomas Geisbert/University of Texas Medical Branch)
How is it treated?
There is currently no vaccine for Marburg and no therapeutics to treat it. But patients can be helped."They need supportive care," Bogoch said, including intravenous fluids, as well as electrolyte balance and monitoring. "That can significantly lower the mortality rate," he said.
Where are the confirmed cases now?
Cases connected to the current outbreak in Equatorial Guinea were first detected in the northern province of Kie-Ntem, near the border with Cameroon.The outbreak was confirmed after samples were sent to a lab in Senegal. Suspected cases from Cameroon and Gabon were also investigated but found not to be Marburg, WHO said.
But that doesn't mean there aren't more Marburg cases.
"When new diseases appear in new locations, we are often just seeing a piece of the picture," said Dr. Kamran Khan, the founder and CEO of Toronto-based BlueDot, a company that tracks infectious diseases around the world.
He said there are probably more cases and more contacts than the official numbers would indicate, noting that Equatorial Guinea is one of the most resource limited countries in the world.
"Its capabilities in terms of its health-care system, its public health infrastructure for countering an outbreak, are pretty limited," he said.
WHO said it is sending medical experts to help local officials in Equatorial Guinea, along with protective equipment for hundreds of workers.
"Surveillance in the field has been intensified," said George Ameh, WHO's country representative in Equatorial Guinea.
"Contact tracing, as you know, is a cornerstone of the response. We have ... redeployed the COVID-19 teams that were there for contact tracing and quickly retrofitted them to really help us out."
WHO director general Tedros Adhanom Ghebreyesus said the agency is also supporting the governments of Cameroon and Gabon "to prepare, to rapidly detect, isolate and provide care for any suspected cases."
WATCH | WHO is deploying teams to Equatorial Guinea to deal with Marburg outbreak:
Trying to contain the Marburg virus
1 month agoDuration 0:56
WHO director general Tedros Adhanom Ghebreyesus says the agency is sending teams and supplies to the Central African country of Equatorial Guinea to try to contain the current outbreak of the deadly Marburg virus.
Is there a concern about spread?
The current Marburg outbreak appears to be regional, but Bogoch notes that infection on one part of the Earth can quickly land on another part in a very short time frame."We saw that with, for example, the West African Ebola virus epidemic — which started off as a very small outbreak, turned into a multi-country, multi-year outbreak that took a long time to get under control."
Khan of BlueDot said Equatorial Guinea is going to need international assistance to be able to get ahead of this outbreak. "Today, it's a concern for the region and some of the neighbouring countries. But if we don't get ahead of this, this could become a broader concern for the global community."
Should people living in Canada be worried?
There's probably no immediate concern about a case of Marburg being found in Canada."I think this is important for Canadians to understand that the likelihood of a case of Marburg showing up in Canada right now is exceedingly low," Kahn said. But he said it's important to be aware of the larger issue — which is that "there are more outbreaks appearing in the world today, they are becoming larger, they are becoming more dangerous and disruptive."
Bogoch said while the current Marburg outbreak is small, now is the time to jump on it so that it doesn't expand.
--------------------------------------------------------
Well, its already to Tanzania, but obviously the Canadian press weren't on top of how our astute virologists saw this coming and were doing the first vaccine clinical trials in January. And the head dude at Siemens was appropriately proud of this as can be seen in his January 25 article above.
Just amazing, almost like these guys had a crystal ball. Thank goodness the WHO is finally (or will be within a week or so) in charge of everything so we don't have to worry about local bureacracies or even the wisdom of supersmart Joe BIden interfering one bit!
Last edited: