This brass is once fired brass. It has been cleaned, resized, swaged etc. I weighed the brass twice. Used denatured alcohol (density weight of 0.794) and converted to H20 weight. This is a sample of 100 pieces.
Filled to the top as shown in this picture:

I made every effort to insure that everyone was filled to this level.
Here are the results:
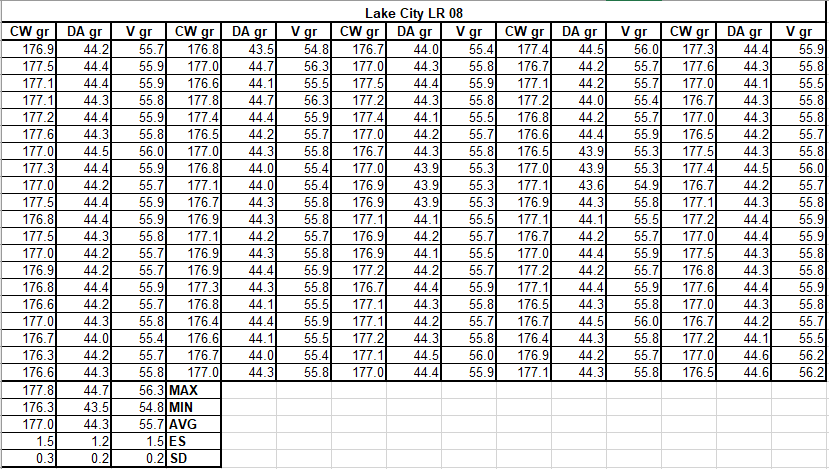
CW: case weight
DA: denatured alcohol
V: volume
The math I used:
Density of H2O = 1g/mL
Density of Denatured Alcohol = 0.794g/mL
Density of IMR 4064 = 0.0745cm3
Density of Varget = 0.0731cm3
1mL = 1/1000mL
1mL = 1cc (cubic centimeter)
1 pound (#) = 453.592 grams (g) (453592mg)
1 pound (#) = 7000 grains (gr)
Weight of 1 grain in grams = 453.592 / 7000 = 1gr = 0.06479886g (64.8mg)
1 gram (1000mg) = 15.4323703 grains
grams * 15.4323703 = grains
Grains / 15.4323703 = grams
Case weight with media – case weight empty = case volume in grains ETOH
Case volume in grains ETOH / 0.794g/ml = case volume in gr H2O
Example: 225gr – 175gr = 50gr ETOH / 0.794 = 62.9722922gr H2O
Conversion to cc/mL:
Xgr H2O * 0.06479886L/gr H2O = YmL (formula used in some auto powder loaders to determine powder drop)
Example 1: 56.5gr H2O * 0.06479886L/gr H2O = 3.66113559ml/cc
Example 2: 3.66113559mL / 0.06479886L/gr H2O = 56.5gr
Next up is to do some alpha munitions 308 brass. Just waiting to finish fire forming some. I will compare 100 fired once and 100 virgin pieces.
Filled to the top as shown in this picture:
I made every effort to insure that everyone was filled to this level.
Here are the results:
CW: case weight
DA: denatured alcohol
V: volume
The math I used:
Case Volume
Density of H2O = 1g/mL
Density of Denatured Alcohol = 0.794g/mL
Density of IMR 4064 = 0.0745cm3
Density of Varget = 0.0731cm3
1mL = 1/1000mL
1mL = 1cc (cubic centimeter)
1 pound (#) = 453.592 grams (g) (453592mg)
1 pound (#) = 7000 grains (gr)
Weight of 1 grain in grams = 453.592 / 7000 = 1gr = 0.06479886g (64.8mg)
1 gram (1000mg) = 15.4323703 grains
grams * 15.4323703 = grains
Grains / 15.4323703 = grams
Case weight with media – case weight empty = case volume in grains ETOH
Case volume in grains ETOH / 0.794g/ml = case volume in gr H2O
Example: 225gr – 175gr = 50gr ETOH / 0.794 = 62.9722922gr H2O
Conversion to cc/mL:
Xgr H2O * 0.06479886L/gr H2O = YmL (formula used in some auto powder loaders to determine powder drop)
Example 1: 56.5gr H2O * 0.06479886L/gr H2O = 3.66113559ml/cc
Example 2: 3.66113559mL / 0.06479886L/gr H2O = 56.5gr
Next up is to do some alpha munitions 308 brass. Just waiting to finish fire forming some. I will compare 100 fired once and 100 virgin pieces.
Last edited:

