Join the Hide community
Get access to live stream, lessons, the post exchange, and chat with other snipers.
Register
Download Gravity Ballistics
Get help to accurately calculate and scope your sniper rifle using real shooting data.

Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
PortaJohn
- Thread starter Lowlight
- Start date
-
- Tags
- sniper's hide
Who has information about VAIDS?
If your bum hurts it's the other one.
You know that from experience?If your bum hurts it's the other one.
confederate flag at convoy was government plant, with .gov photographer following him around.


Someone claiming this was overheard on police scanner:
Also saw report that rubber bullets and tear gas are greenlit.
"They just said octranspo is sending buses downtown after their shift. Usually they use those for mass arrest"
Also saw report that rubber bullets and tear gas are greenlit.
Someone claiming this was overheard on police scanner:
"They just said octranspo is sending buses downtown after their shift. Usually they use those for mass arrest"
Also saw report that rubber bullets and tear gas are greenlit.
Canada and then the fucking entire WORLD would ERUPT

Spice company that called Republicans racist begs for gift card purchases after losing customers
A Wisconsin-based spice company that made headlines earlier this month when its CEO sent an email to customers accusing Republicans of racism is now asking people to buy gift cards after hemorrhaging tens of thousands of customers.
Another poignant response in song form:
Spice company that called Republicans racist begs for gift card purchases after losing customers
A Wisconsin-based spice company that made headlines earlier this month when its CEO sent an email to customers accusing Republicans of racism is now asking people to buy gift cards after hemorrhaging tens of thousands of customers.www.foxbusiness.com
Who has information about VAIDS?
I honestly don't like the term "VAIDS" because it imply Vaccines cause the same immunodeficiency as advanced HIV; but mounting evidence is inferring there is some compromise of the immune system from both infection and vaccination. Papers need be done looking at effects on number and function of all WBCs (especially lymphocytes) pre- and post- infection and vaccination. The Lancet article cited by the "VAIDS" article online actually come to the conclusion the highest risk group should be boostered.
https://newstarget.com/2021-12-10-vaccine-induced-vaids-rising-mass-covid-vaccination.html
https://www.educationviews.org › vaccine-acquired-immune-deficiency-syndrome-vaids-we-should
This is the Lancet article frequently cited, but VAIDS isn't mentioned in the abstract.
Effectiveness of Covid-19 Vaccination Against Risk of Symptomatic Infection, Hospitalization, and Death Up to 9 Months: A Swedish Total-Population Cohort Study
34 Pages Posted: 25 Oct 2021Peter Nordström
University of Umea - Unit of Geriatric MedicineMarcel Ballin
University of UmeaAnna Nordström
University of UmeaMore...
Abstract
Background: Whether vaccine effectiveness against Coronavirus disease 2019 (Covid-19) lasts longer than 6 months is unclear.Methods: A retrospective cohort study was conducted using Swedish nationwide registries. The cohort comprised 842,974 pairs (N=1,684,958), including individuals vaccinated with 2 doses of ChAdOx1 nCoV-19, mRNA-1273, or BNT162b2, and matched unvaccinated individuals. Cases of symptomatic infection and severe Covid-19 (hospitalization or 30-day mortality after confirmed infection) were collected from 12 January to 4 October 2021.
Findings: Vaccine effectiveness of BNT162b2 against infection waned progressively from 92% (95% CI, 92-93, P<0·001) at day 15-30 to 47% (95% CI, 39-55, P<0·001) at day 121-180, and from day 211 and onwards no effectiveness could be detected (23%; 95% CI, -2-41, P=0·07). The effectiveness waned slightly slower for mRNA-1273, being estimated to 59% (95% CI, 18-79) from day 181 and onwards. In contrast, effectiveness of ChAdOx1 nCoV-19 was generally lower and waned faster, with no effectiveness detected from day 121 and onwards (-19%, 95% CI, -97-28), whereas effectiveness from heterologous ChAdOx1 nCoV-19 / mRNA was maintained from 121 days and onwards (66%; 95% CI, 41-80). Overall, vaccine effectiveness was lower and waned faster among men and older individuals. For the outcome severe Covid-19, effectiveness waned from 89% (95% CI, 82-93, P<0·001) at day 15-30 to 42% (95% CI, -35-75, P=0·21) from day 181 and onwards, with sensitivity analyses showing notable waning among men, older frail individuals, and individuals with comorbidities.
Interpretation: Vaccine effectiveness against symptomatic Covid-19 infection wanes progressively over time across all subgroups, but at different rate according to type of vaccine, and faster for men and older frail individuals. The effectiveness against severe illness seems to remain high through 9 months, although not for men, older frail individuals, and individuals with comorbidities. This strengthens the evidence-based rationale for administration of a third booster dose.
Funding: None to declare.
Declaration of Interest: None to declare.
Ethical Approval: This study was approved by the Swedish Ethical Review Authority (number 495/2021)
Keywords: Covid-19 infection, Vaccination
Suggested Citation:
Nordström, Peter and Ballin, Marcel and Nordström, Anna, Effectiveness of Covid-19 Vaccination Against Risk of Symptomatic Infection, Hospitalization, and Death Up to 9 Months: A Swedish Total-Population Cohort Study. Available at SSRN: https://ssrn.com/abstract=3949410 or http://dx.doi.org/10.2139/ssrn.3949410
This article just released on Friday from NEJM (biggest US journal) about autoimmune antibody potential rom both COVID infection and vaccination.:
A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination
List of authors.- William J. Murphy, Ph.D.,
- and Dan L. Longo, M.D.
- Article
- Figures/Media
The pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is incompletely understood, with its effects on multiple organ systems1 and the syndrome of “long Covid” occurring long after the resolution of infection.2 The development of multiple efficacious vaccines has been critical in the control of the pandemic, but their efficacy has been limited by the appearance of viral variants, and the vaccines can be associated with rare off-target or toxic effects, including allergic reactions, myocarditis, and immune-mediated thrombosis and thrombocytopenia in some healthy adults. Many of these phenomena are likely to be immune-mediated.3 How can we understand this diversity in immune responses in different persons?The Clinical Implications of Basic Research series has focused on highlighting laboratory research that could lead to advances in clinical therapeutics. However, the path between the laboratory and the bedside runs both ways: clinical observations often pose new questions for laboratory investigations that then lead back to the clinic. One of a series of occasional articles drawing attention to the bedside-to-bench flow of information is presented here, under the Basic Implications of Clinical Observations rubric. We hope our readers will enjoy these stories of discovery, and we invite them to submit their own examples of clinical findings that have led to insights in basic science.
Figure 1.

One way of thinking about the complexity of the immune response is through the lens of anti-idiotype immune responses. The Network Hypothesis, formulated in 1974 by Niels Jerne, described a mechanism by which the antibody responses to an antigen themselves induced downstream antibody responses against the antigen-specific antibody.4 Every antibody that is induced and specific for an antigen (termed “Ab1” antibody) has immunogenic regions, particularly in their variable-region antigen-binding domains, that are unique as a result of genetic recombination of immunoglobulin variable, diversity, and joining (VDJ) genes; VDJ recombination results in new and therefore immunogenic amino acid sequences called idiotopes, which are then capable of inducing specific antibodies against Ab1 antibodies as a form of down-regulation. A similar paradigm has been proposed for T cells. However, these regulatory immune responses are also capable of doing much more. The paratopes, or antigen-binding domains, of some of the resulting anti-idiotype (or “Ab2”) antibodies that are specific for Ab1 can structurally resemble that of the original antigens themselves. Thus, the Ab2 antigen-binding region can potentially represent an exact mirror image of the initial targeted antigen in the Ab1 response, and Ab2 antibodies have even been examined for potential use as a surrogate for the antigen in vaccine studies. However, as a result of this mimicry, Ab2 antibodies also have the potential to bind the same receptor that the original antigen was targeting (Figure 1). Ab2 antibodies binding to the original receptor on normal cells therefore have the potential to mediate profound effects on the cell that could result in pathologic changes, particularly in the long term — long after the original antigen itself has disappeared.
This aspect of regulation of immune-cell responses was postulated by Plotz in 1983 as a possible cause of autoimmunity arising after viral infection5 and has since been supported experimentally by direct transfer of anti-idiotype antibodies. Ab2 antibodies generated against the enterovirus coxsackievirus B3 in mice can bind myocyte antigens, resulting in autoimmune myocarditis,6 and anti-idiotype responses can act as acetylcholine receptor agonists, leading to myasthenia gravis symptoms in rabbits.7 In addition, by displaying the mirror image of the viral antigen, Ab2 alone can even mimic the deleterious effects of the virus particle itself, as has been shown with bovine viral diarrhea virus antigen.8
For SARS-CoV-2 infection, attention centers on the spike (S) protein and its critical use of the angiotensin-converting–enzyme 2 (ACE2) receptor to gain entry into the cell. Given its critical role in regulating angiotensin responses, many physiological effects can be influenced by ACE2 engagement.9 The S protein itself has a direct effect on suppressing ACE2 signaling by a variety of mechanisms and can also directly trigger toll-like receptors and induce inflammatory cytokines.10Anti-idiotype responses may affect ACE2 function, resulting in similar effects. However, preclinical and clinical assessments of antibody responses to SARS-CoV-2 vaccines have focused solely on Ab1 responses and virus-neutralizing efficacy. The delineation of potential anti-idiotype responses has inherent difficulties because of the polyclonal nature of responses, dynamic kinetics, and the concurrent presence of both Ab1 and Ab2 antibodies. Furthermore, ACE2 expression within cells and tissues can be variable. The different vaccine constructs (RNA, DNA, adenoviral, and protein) are also likely to have differential effects on Ab2 induction or in the mediation of vaccine effects that differ from responses to infection. Some off-target effects may not be directly linked to Ab2 responses. The association of thrombotic events with some SARS-CoV-2 vaccines in young women and the etiologic role of anti–platelet factor 4–polyanion antibodies may be the result of the adenoviral vector. However, the reported occurrence of myocarditis after vaccine administration bears striking similarities to the myocarditis associated with Ab2 antibodies induced after some viral infections.6 Ab2 antibodies could also mediate neurologic effects of SARS-CoV-2 infection or vaccines, given the expression of ACE2 on neuronal tissues, the specific neuropathologic effects of SARS-CoV-2 infection,11 and the similarity of these effects to Ab2-mediated neurologic effects observed in other viral models.
It would therefore be prudent to fully characterize all antibody and T-cell responses to the virus and the vaccines, including Ab2 responses over time. Using huACE2 transgenic mice and crossing them with strains that are predisposed to autoimmunity or other human pathologic conditions can also provide important insights. An understanding of potential Ab2 responses may also provide insights into Ab1 maintenance and efficacy and into the application of antibody-based therapeutic agents. However, much more basic science research is needed to determine the potential role idiotype-based immunoregulation of both humoral and cell-mediated responses may play both in antiviral efficacy and in unwanted side effects of both SARS-CoV-2 infection and the vaccines that protect us from it.
Disclosure forms provided by the authors are available at NEJM.org.
This article was published on November 24, 2021, at NEJM.org.
Author Affiliations
From the Departments of Dermatology and Internal Medicine, Division of Hematology and Oncology, University of California, Davis, Sacramento (W.J.M.).
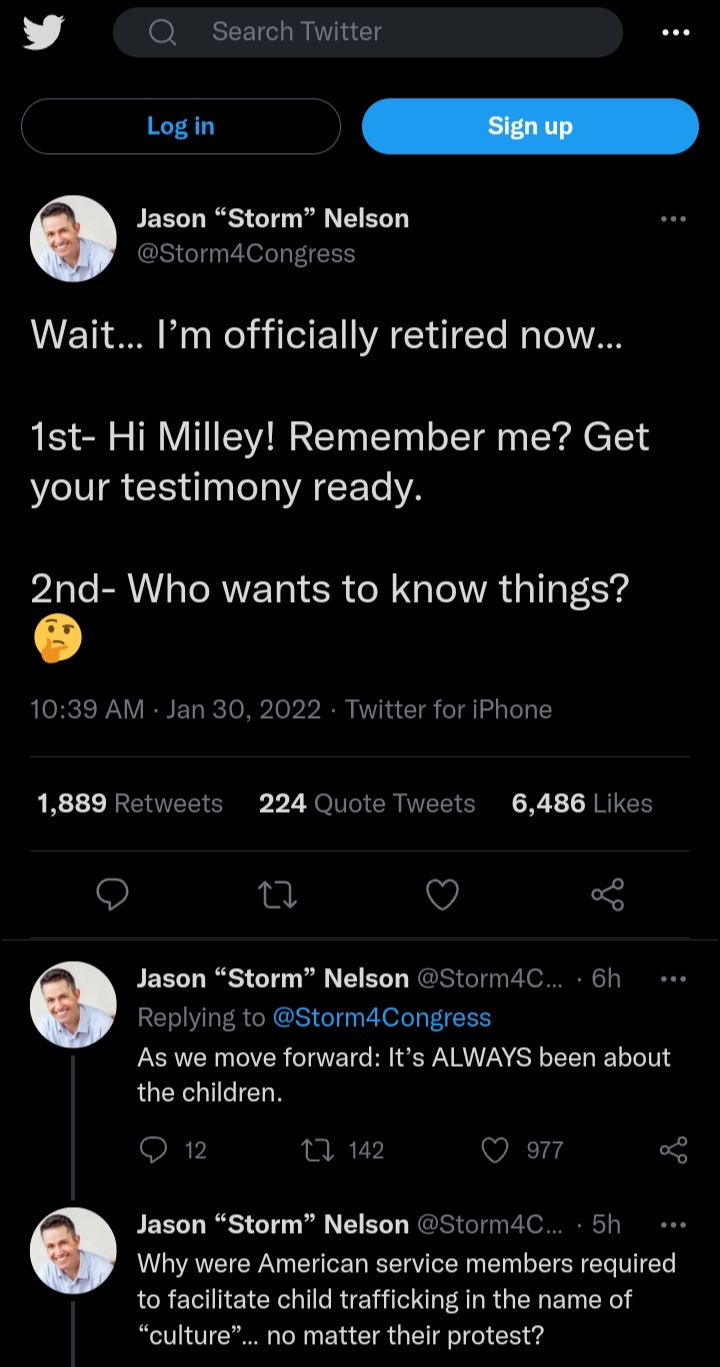
I defintely want to know more here

LAWRENCE SELLIN: China’s Military May Be Making a New Human-Infecting Coronavirus Deadlier than COVID-19 | The Gateway Pundit | by Joe Hoft
China’s military may be making a new human-infecting coronavirus deadlier than COVID-19 China caused the COVID-19 pandemic by artificially manipulating the structure of a natural bat coronavirus to make it infectious to humans.
at this point we need to either nuke China back to Stone Age or some kind of never before seen cyber attack that would do same thing.
Don't be the least bit surprised when commies get "forced" to commie for the "public good" against the "extremist" protesters trying to "disrupt the government business".
This version of Trudeau may be just under the surface.
"If you think strong men are dangerous, wait until you see what weak men are capable of."
-Jordan Peterson.

LAWRENCE SELLIN: China’s Military May Be Making a New Human-Infecting Coronavirus Deadlier than COVID-19 | The Gateway Pundit | by Joe Hoft
China’s military may be making a new human-infecting coronavirus deadlier than COVID-19 China caused the COVID-19 pandemic by artificially manipulating the structure of a natural bat coronavirus to make it infectious to humans.www.thegatewaypundit.com
at this point we need to either nuke China back to Stone Age or some kind of never before seen cyber attack that would do same thing.
Yo if they seem to be this hell bent on destroying the world they need to get fucking clapped... What would ANYBODY do if they are on a plane 35,000 feet in the air and some maniac is trying to force the emergency door open?
Someone claiming this was overheard on police scanner:
"They just said octranspo is sending buses downtown after their shift. Usually they use those for mass arrest"
Also saw report that rubber bullets and tear gas are greenlit.
Depending on just how much force is used by the state npcs, these buses should be torched as soon as they show up. If NPCs start shooting, then it is balls out time. Enough is fucking enough. Kids are dying from these poison shots.

The Pressure Campaign on Spotify to Remove Joe Rogan Reveals the Religion of Liberals: Censorship
All factions, at certain points, succumb to the impulse to censor. But for the Democratic Party's liberal adherents, silencing their adversaries has become their primary project.

EXCLUSIVE: Oath Keepers Founder Stewart Rhodes Moved to the Cimarron Facility in Cushing, Oklahoma | The Gateway Pundit | by Jim Hoft
EXCLUSIVE: Oath Keepers Founder Stewart Rhodes Moved to the Cimarron Facility in Cushing, Oklahoma The Gateway Pundit

EXCLUSIVE: Last Week's Court Ruling in Pennsylvania Means 40% of 2020 Ballots Unconstitutional -- Without These Ballots President Trump Crushed Biden by a 2 to 1 Ratio in the State! | The Gateway Pundit | by Joe Hoft
Attorney Wally Zimolong in Pennsylvania won a historic case last week in the Commonwealth Court. The justices agreed that the state’s constitution does not provide for ‘no excuse’ mail-in balloting. Steve Bannon’s War Room invited attorney Wally Zimolong on to discuss the use of absentee...
I defintely want to know more here
I don't know many details but it goes something like this: Milley was in charge of a FOB in Afghanistan, that FOB was running drugs and human trafficking. It appears that Milley is implicated and this guy is now out so he plans to expose it. There is another guy that was trying to expose it but .gov goons went after him and I believe he's locked up.
What percentage of people with "long COVID" were treated with remdesvir? What percentage of people with "long COVID" received no treatment until they had pneumonia? What percentage of people with "long covid" are vaccinated? What percentage of people with long COVID are suffering from conditions that were found when they had COVID?I honestly don't like the term "VAIDS" because it imply Vaccines cause the same immunodeficiency as advanced HIV; but mounting evidence is inferring there is some compromise of the immune system from both infection and vaccination. Papers need be done looking at effects on number and function of all WBCs (especially lymphocytes) pre- and post- infection and vaccination. The Lancet article cited by the "VAIDS" article online actually come to the conclusion the highest risk group should be boostered.
https://newstarget.com/2021-12-10-vaccine-induced-vaids-rising-mass-covid-vaccination.html
https://www.educationviews.org › vaccine-acquired-immune-deficiency-syndrome-vaids-we-should
This is the Lancet article frequently cited, but VAIDS isn't mentioned in the abstract.
Effectiveness of Covid-19 Vaccination Against Risk of Symptomatic Infection, Hospitalization, and Death Up to 9 Months: A Swedish Total-Population Cohort Study
34 Pages Posted: 25 Oct 2021
Peter Nordström
University of Umea - Unit of Geriatric Medicine
Marcel Ballin
University of Umea
Anna Nordström
University of Umea
More...
Abstract
Background: Whether vaccine effectiveness against Coronavirus disease 2019 (Covid-19) lasts longer than 6 months is unclear.
Methods: A retrospective cohort study was conducted using Swedish nationwide registries. The cohort comprised 842,974 pairs (N=1,684,958), including individuals vaccinated with 2 doses of ChAdOx1 nCoV-19, mRNA-1273, or BNT162b2, and matched unvaccinated individuals. Cases of symptomatic infection and severe Covid-19 (hospitalization or 30-day mortality after confirmed infection) were collected from 12 January to 4 October 2021.
Findings: Vaccine effectiveness of BNT162b2 against infection waned progressively from 92% (95% CI, 92-93, P<0·001) at day 15-30 to 47% (95% CI, 39-55, P<0·001) at day 121-180, and from day 211 and onwards no effectiveness could be detected (23%; 95% CI, -2-41, P=0·07). The effectiveness waned slightly slower for mRNA-1273, being estimated to 59% (95% CI, 18-79) from day 181 and onwards. In contrast, effectiveness of ChAdOx1 nCoV-19 was generally lower and waned faster, with no effectiveness detected from day 121 and onwards (-19%, 95% CI, -97-28), whereas effectiveness from heterologous ChAdOx1 nCoV-19 / mRNA was maintained from 121 days and onwards (66%; 95% CI, 41-80). Overall, vaccine effectiveness was lower and waned faster among men and older individuals. For the outcome severe Covid-19, effectiveness waned from 89% (95% CI, 82-93, P<0·001) at day 15-30 to 42% (95% CI, -35-75, P=0·21) from day 181 and onwards, with sensitivity analyses showing notable waning among men, older frail individuals, and individuals with comorbidities.
Interpretation: Vaccine effectiveness against symptomatic Covid-19 infection wanes progressively over time across all subgroups, but at different rate according to type of vaccine, and faster for men and older frail individuals. The effectiveness against severe illness seems to remain high through 9 months, although not for men, older frail individuals, and individuals with comorbidities. This strengthens the evidence-based rationale for administration of a third booster dose.
Funding: None to declare.
Declaration of Interest: None to declare.
Ethical Approval: This study was approved by the Swedish Ethical Review Authority (number 495/2021)
Keywords: Covid-19 infection, Vaccination
Suggested Citation:
Nordström, Peter and Ballin, Marcel and Nordström, Anna, Effectiveness of Covid-19 Vaccination Against Risk of Symptomatic Infection, Hospitalization, and Death Up to 9 Months: A Swedish Total-Population Cohort Study. Available at SSRN: https://ssrn.com/abstract=3949410 or http://dx.doi.org/10.2139/ssrn.3949410
This article just released on Friday from NEJM (biggest US journal) about autoimmune antibody potential rom both COVID infection and vaccination.:
A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination
List of authors.
Metrics
- William J. Murphy, Ph.D.,
- and Dan L. Longo, M.D.
- Article
- Figures/Media
The pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is incompletely understood, with its effects on multiple organ systems1 and the syndrome of “long Covid” occurring long after the resolution of infection.2 The development of multiple efficacious vaccines has been critical in the control of the pandemic, but their efficacy has been limited by the appearance of viral variants, and the vaccines can be associated with rare off-target or toxic effects, including allergic reactions, myocarditis, and immune-mediated thrombosis and thrombocytopenia in some healthy adults. Many of these phenomena are likely to be immune-mediated.3 How can we understand this diversity in immune responses in different persons?
Figure 1.Anti-idiotype Antibodies and SARS-CoV-2.
One way of thinking about the complexity of the immune response is through the lens of anti-idiotype immune responses. The Network Hypothesis, formulated in 1974 by Niels Jerne, described a mechanism by which the antibody responses to an antigen themselves induced downstream antibody responses against the antigen-specific antibody.4 Every antibody that is induced and specific for an antigen (termed “Ab1” antibody) has immunogenic regions, particularly in their variable-region antigen-binding domains, that are unique as a result of genetic recombination of immunoglobulin variable, diversity, and joining (VDJ) genes; VDJ recombination results in new and therefore immunogenic amino acid sequences called idiotopes, which are then capable of inducing specific antibodies against Ab1 antibodies as a form of down-regulation. A similar paradigm has been proposed for T cells. However, these regulatory immune responses are also capable of doing much more. The paratopes, or antigen-binding domains, of some of the resulting anti-idiotype (or “Ab2”) antibodies that are specific for Ab1 can structurally resemble that of the original antigens themselves. Thus, the Ab2 antigen-binding region can potentially represent an exact mirror image of the initial targeted antigen in the Ab1 response, and Ab2 antibodies have even been examined for potential use as a surrogate for the antigen in vaccine studies. However, as a result of this mimicry, Ab2 antibodies also have the potential to bind the same receptor that the original antigen was targeting (Figure 1). Ab2 antibodies binding to the original receptor on normal cells therefore have the potential to mediate profound effects on the cell that could result in pathologic changes, particularly in the long term — long after the original antigen itself has disappeared.
This aspect of regulation of immune-cell responses was postulated by Plotz in 1983 as a possible cause of autoimmunity arising after viral infection5 and has since been supported experimentally by direct transfer of anti-idiotype antibodies. Ab2 antibodies generated against the enterovirus coxsackievirus B3 in mice can bind myocyte antigens, resulting in autoimmune myocarditis,6 and anti-idiotype responses can act as acetylcholine receptor agonists, leading to myasthenia gravis symptoms in rabbits.7 In addition, by displaying the mirror image of the viral antigen, Ab2 alone can even mimic the deleterious effects of the virus particle itself, as has been shown with bovine viral diarrhea virus antigen.8
For SARS-CoV-2 infection, attention centers on the spike (S) protein and its critical use of the angiotensin-converting–enzyme 2 (ACE2) receptor to gain entry into the cell. Given its critical role in regulating angiotensin responses, many physiological effects can be influenced by ACE2 engagement.9 The S protein itself has a direct effect on suppressing ACE2 signaling by a variety of mechanisms and can also directly trigger toll-like receptors and induce inflammatory cytokines.10Anti-idiotype responses may affect ACE2 function, resulting in similar effects. However, preclinical and clinical assessments of antibody responses to SARS-CoV-2 vaccines have focused solely on Ab1 responses and virus-neutralizing efficacy. The delineation of potential anti-idiotype responses has inherent difficulties because of the polyclonal nature of responses, dynamic kinetics, and the concurrent presence of both Ab1 and Ab2 antibodies. Furthermore, ACE2 expression within cells and tissues can be variable. The different vaccine constructs (RNA, DNA, adenoviral, and protein) are also likely to have differential effects on Ab2 induction or in the mediation of vaccine effects that differ from responses to infection. Some off-target effects may not be directly linked to Ab2 responses. The association of thrombotic events with some SARS-CoV-2 vaccines in young women and the etiologic role of anti–platelet factor 4–polyanion antibodies may be the result of the adenoviral vector. However, the reported occurrence of myocarditis after vaccine administration bears striking similarities to the myocarditis associated with Ab2 antibodies induced after some viral infections.6 Ab2 antibodies could also mediate neurologic effects of SARS-CoV-2 infection or vaccines, given the expression of ACE2 on neuronal tissues, the specific neuropathologic effects of SARS-CoV-2 infection,11 and the similarity of these effects to Ab2-mediated neurologic effects observed in other viral models.
It would therefore be prudent to fully characterize all antibody and T-cell responses to the virus and the vaccines, including Ab2 responses over time. Using huACE2 transgenic mice and crossing them with strains that are predisposed to autoimmunity or other human pathologic conditions can also provide important insights. An understanding of potential Ab2 responses may also provide insights into Ab1 maintenance and efficacy and into the application of antibody-based therapeutic agents. However, much more basic science research is needed to determine the potential role idiotype-based immunoregulation of both humoral and cell-mediated responses may play both in antiviral efficacy and in unwanted side effects of both SARS-CoV-2 infection and the vaccines that protect us from it.
Disclosure forms provided by the authors are available at NEJM.org.
This article was published on November 24, 2021, at NEJM.org.
Author Affiliations
From the Departments of Dermatology and Internal Medicine, Division of Hematology and Oncology, University of California, Davis, Sacramento (W.J.M.).
I would refer to this paragraph as towing the line.
The development of multiple efficacious vaccines has been critical in the control of the pandemic, but their efficacy has been limited by the appearance of viral variants, and the vaccines can be associated with rare off-target or toxic effects, including allergic reactions, myocarditis, and immune-mediated thrombosis and thrombocytopenia in some healthy adults.

Why the Masked and the Unmasked Have Disdain for Each Other
Among the many unbridgeable divides between Americans is a completely antithetical view of mask wear
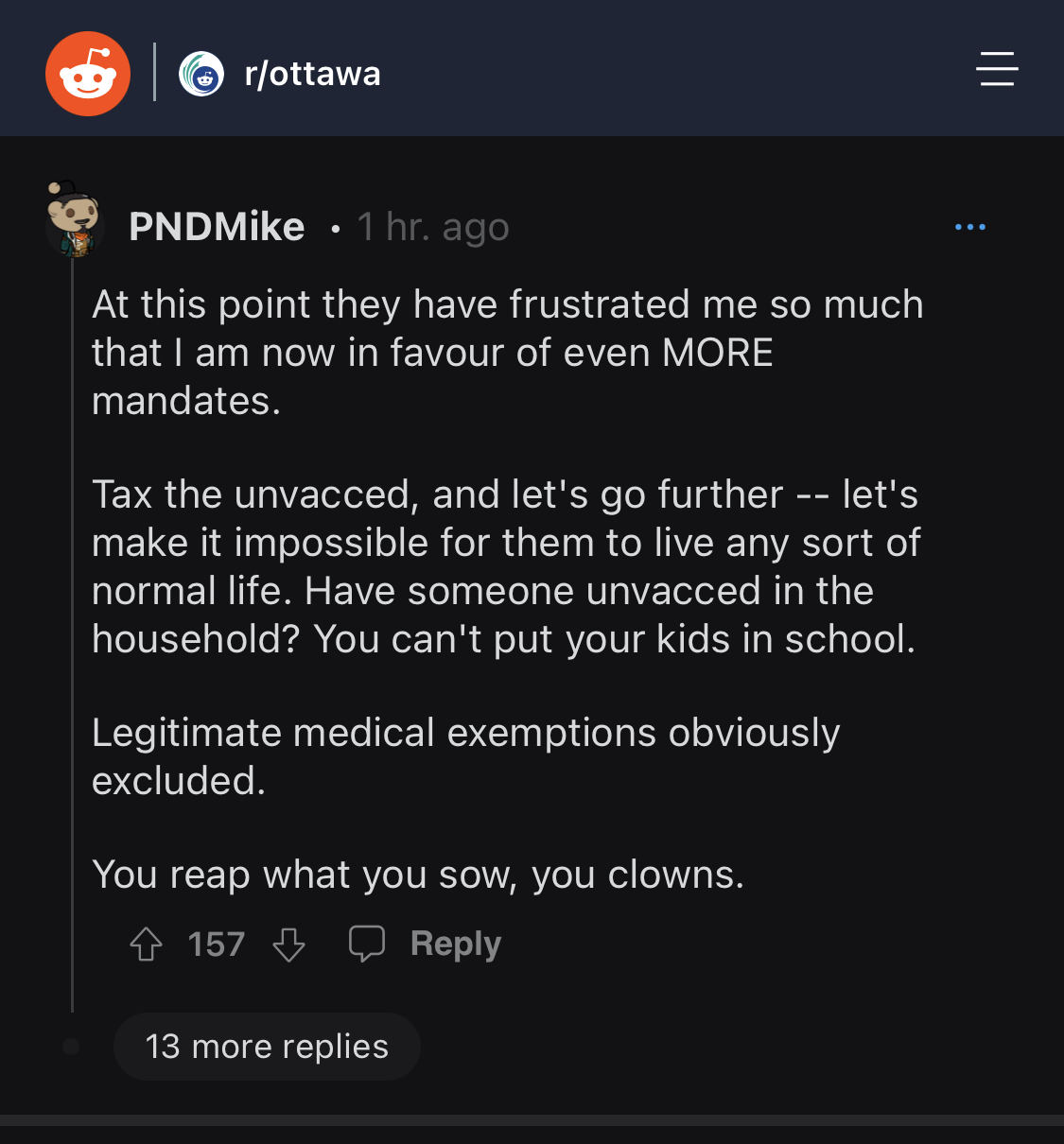
Any doubt of how evil these people are should now fully be erased. It's now more critical than ever that this movement be successful. Pure tyranny is what these people want.the new nazis have a solution for canada

i tried to suffer through that post….the more i read, the more it seemed like propaganda bullshit. like we should believe anything that comes from these fucking fools anymore.What percentage of people with "long COVID" were treated with remdesvir? What percentage of people with "long COVID" received no treatment until they had pneumonia? What percentage of people with "long covid" are vaccinated? What percentage of people with long COVID are suffering from conditions that were found when they had COVID?
I would refer to this paragraph as towing the line.
The development of multiple efficacious vaccines has been critical in the control of the pandemic, but their efficacy has been limited by the appearance of viral variants, and the vaccines can be associated with rare off-target or toxic effects, including allergic reactions, myocarditis, and immune-mediated thrombosis and thrombocytopenia in some healthy adults.
Think things will change IF (and it's a big if) the Pubics get back into "control" (not leadership, for sure).

 redstate.com
redstate.com

Democrats Go Scorched Earth on Redistricting While Republicans Curl Into the Fetal Position
With a mid-term election less than a year away, states are scrambling to finish up the redistricting
/cloudfront-us-east-2.images.arcpublishing.com/reuters/ILJZNGVNLRPQ7BLVHAQCU7UW3I.jpg)
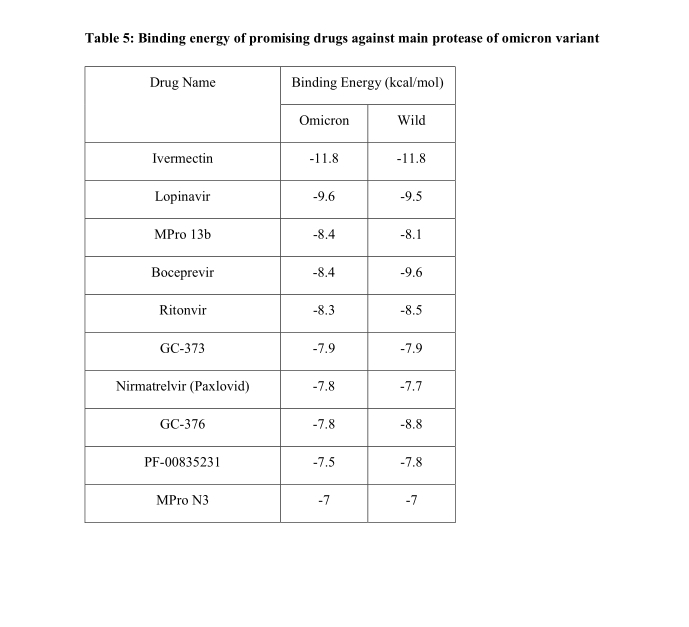
Ivermectin shows ‘antiviral effect’ against COVID, Japanese company says
Japanese trading and pharmaceuticals company Kowa Co Ltd on Monday said that anti-parasite drug ivermectin showed an "antiviral effect" against Omicron and other coronavirus variants in joint non-clinical research.
also
Last edited:
Three cartoons that pretty much describe our current situation

reps need to go full bore on offensiveThink things will change IF (and it's a big if) the Pubics get back into "control" (not leadership, for sure).

Democrats Go Scorched Earth on Redistricting While Republicans Curl Into the Fetal Position
With a mid-term election less than a year away, states are scrambling to finish up the redistrictingredstate.com
Probably the most accurate description of far too many American cities today

Sooooo... soccer + cocaine. Got it.
I agree, but they are too emasculated to do that. Worthless.reps need to go full bore on offensive
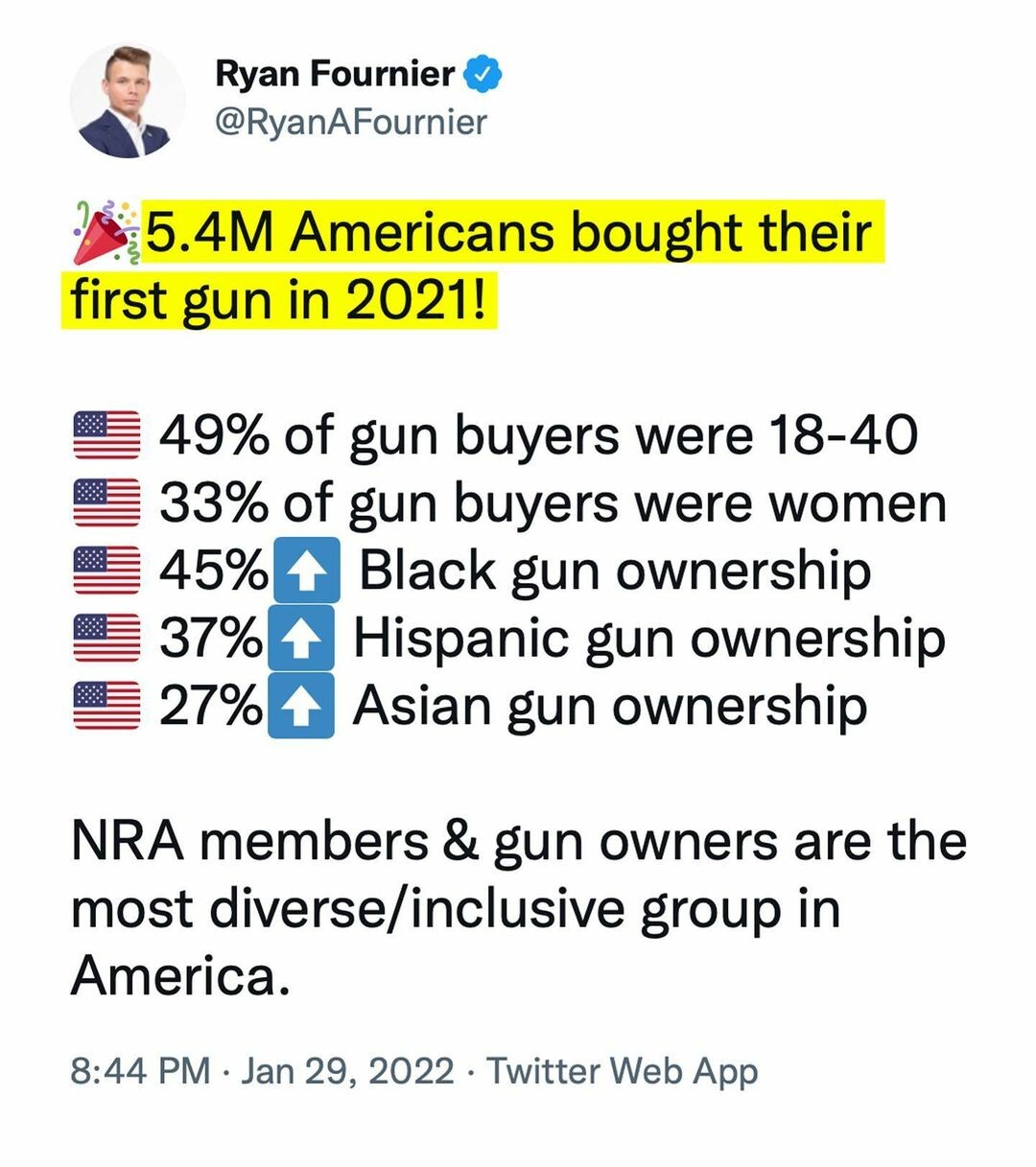
that's the messaging as well as gun control is racist. These two points need to be marketed by GOA and NRA and others.
haven't seen anything on the overnight activities. Did the jackboots start rounding up the truckers?
Watching a live stream now... I don't see anything yethaven't seen anything on the overnight activities. Did the jackboots start rounding up the truckers?
LOUD AS FUCK!!!
Watching a live stream now... I don't see anything yet
LOUD AS FUCK!!!
I hope it remains all shut down and this spreads out of control. I want complete capitulation by the jackboots
I believe the local tow truck drivers are with the protest.haven't seen anything on the overnight activities. Did the jackboots start rounding up the truckers?
Personally I’ve never met anyone with a tow rig big enough to tow a semi that’s a liberal lefty.
If they start arresting people... whos going to move the trucks?
Similar threads
- Replies
- 0
- Views
- 509